Figure 3.
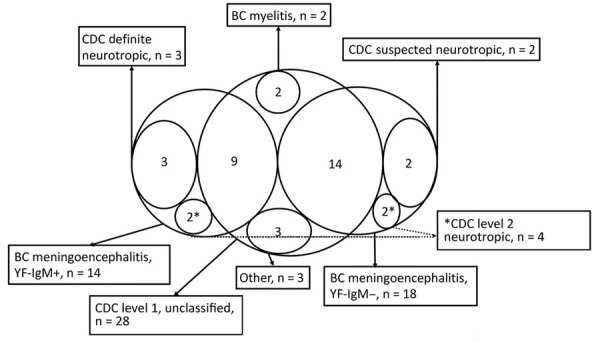
Classification of cases of yellow fever vaccine–associated neurologic disease, São Paulo, Brazil, 2017–2018. Excluded cases, acute disseminated encephalomyelitis cases, and Guillain-Barré syndrome cases not shown. The area with n= 9 represents the intersection between the group "BC meningoencephalitis, YF-IgM+ (reactive CSF-YF-IgM)” and “CDC level 1, unclassified.” The area with n = 14 represents the intersection between the group "BC meningoencephalitis, YF-IgM– (nonconfirmed)" and "CDC level 1, unclassified." BC, Brighton Collaboration criteria; CDC, Centers for Disease Control and Prevention criteria; level 1 unclassified, level 1 neurologic disease not classifiable as level 2; level 2 neurotropic, level 2 neurotropic disease not further classified as suspected or definite neurotropic disease; other, includes atypical yellow fever vaccine–associated neurologic disease (optic neuritis, n = 1; ataxia, n = 1; opsoclonus-myoclonus-ataxia syndrome, n = 1); +, positive.
