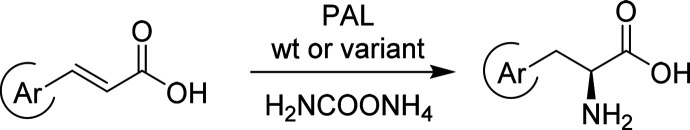
Table 1. AL-Catalyzed Hydroamination of Various Electron-Rich Substituted Cinnamic Acids.
| substrate | Ar substituents | PbPALa conversion (%) | AL-11a conversion (%) |
|---|---|---|---|
| 5a | 2-MeO | 27 | 68 |
| 5b | 3-MeO | 92 | 61 |
| 5c | 4-MeO | 8 | 5 |
| 5d | 3-MeO-4-F | 79 | 91 |
| 5e | 3-MeO-4-Cl | 91 | 59 |
| 5f | 3-F-4-MeO | 40 | 81 |
| 5g | 3-Cl-4-MeO | 38 | 87 |
| 5h | 2-Et | 48 | 91 |
| 5i | 3-Et | 30 | 76 |
| 5j | 4-Et | 48 | 5 |
| 5k | 2,3-(OCH2O) | 92 | 98 |
| 5l | 3,4-(OCH2O) | 3 | 11 |
| 5m | 2,3-(MeO)2 | <1 | 5 |
| 5n | 2,4-(MeO)2 | <1 | <1 |
| 5o | 3,4-(MeO)2 | <1 | 92 |
| 5p | 3,4-(MeO)2-6-F | <1 | 96 |
| 5q | 3,5-(MeO)2 | <1 | 98 |
| 5r | 2,4,6-(MeO)3 | <1 | <1 |
| 5s | 3,4,5-(MeO)3 | <1 | 97 |
| 5t | 2,3,4-(MeO)3 | <1 | 4 |
| 5u | 2,4,5-(MeO)3 | <1 | 73 |
Determined on nonchiral reverse-phase HPLC.