Figure 4.
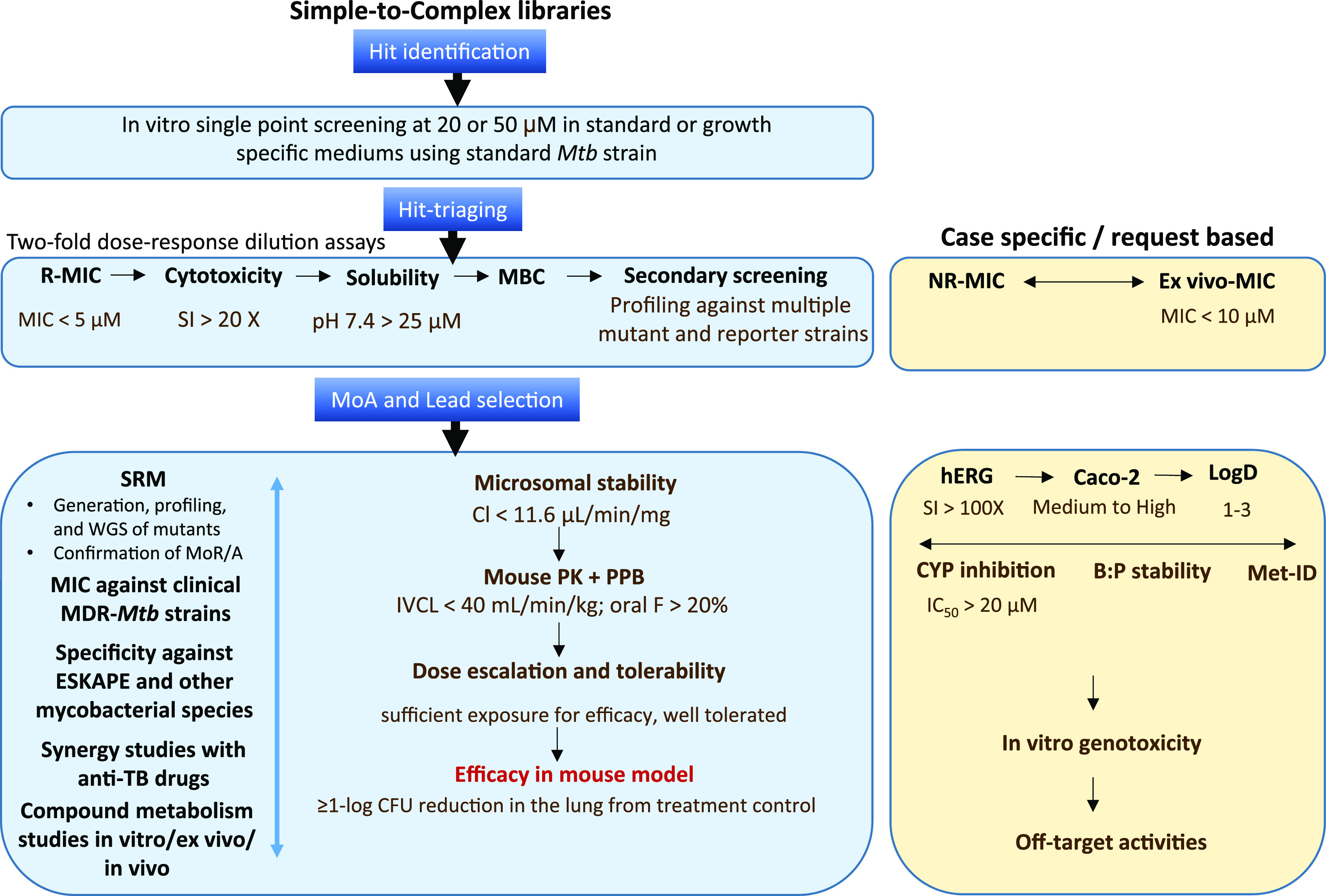
Early drug discovery test-cascade used at the Drug Discovery and Development Centre (H3D). Mtb, M. tuberculosis H37Rv; R, replicating; MIC, minimum inhibitory concentration (7–14 days); cytotoxicity, against VERO (kidney epithelial cells extracted from an African green monkey) and/or CHO (Chinese hamster ovarian) and/or HepG2 (human liver cancer) mammalian cell-lines; solubility, in fasted-state intestinal fluid (FaSSIF) kinetic-solubility assay at pH 6.5 and in PBS at pH 7.4; MBC, minimum bactericidal concentration (7–28 days); NR, nonreplicating, nutrient starvation/hypoxia/4-stress model; ex vivo, using RAW264.7, J774, and/or THP.1 derived macrophages; MoA, mechanism of action; SRM, spontaneous resistant mutant; WGS, whole-genome sequencing; PK, pharmacokinetics; PPB, plasma protein binding; F, bioavailability; CYP, Cytochrome P450; B:P, blood:plasma ratio; MetID, metabolite identification; MoR, mechanism of resistance; SI, selectivity-index; CL, clearance; IVCL, in vivo clearance; genotoxicity, AMES unless the compounds are active against Salmonella, mouse lymphoma, and mouse micronucleation assays; off-target activities, secondary pharmaceutical panels of enzymes, GPCRs, ion channels, receptors, transporters, etc. Dotmatics is used in data management.
