Scheme 1. Synthesis Route toward Pyrimidine and Triazine Derivatives 1–3.
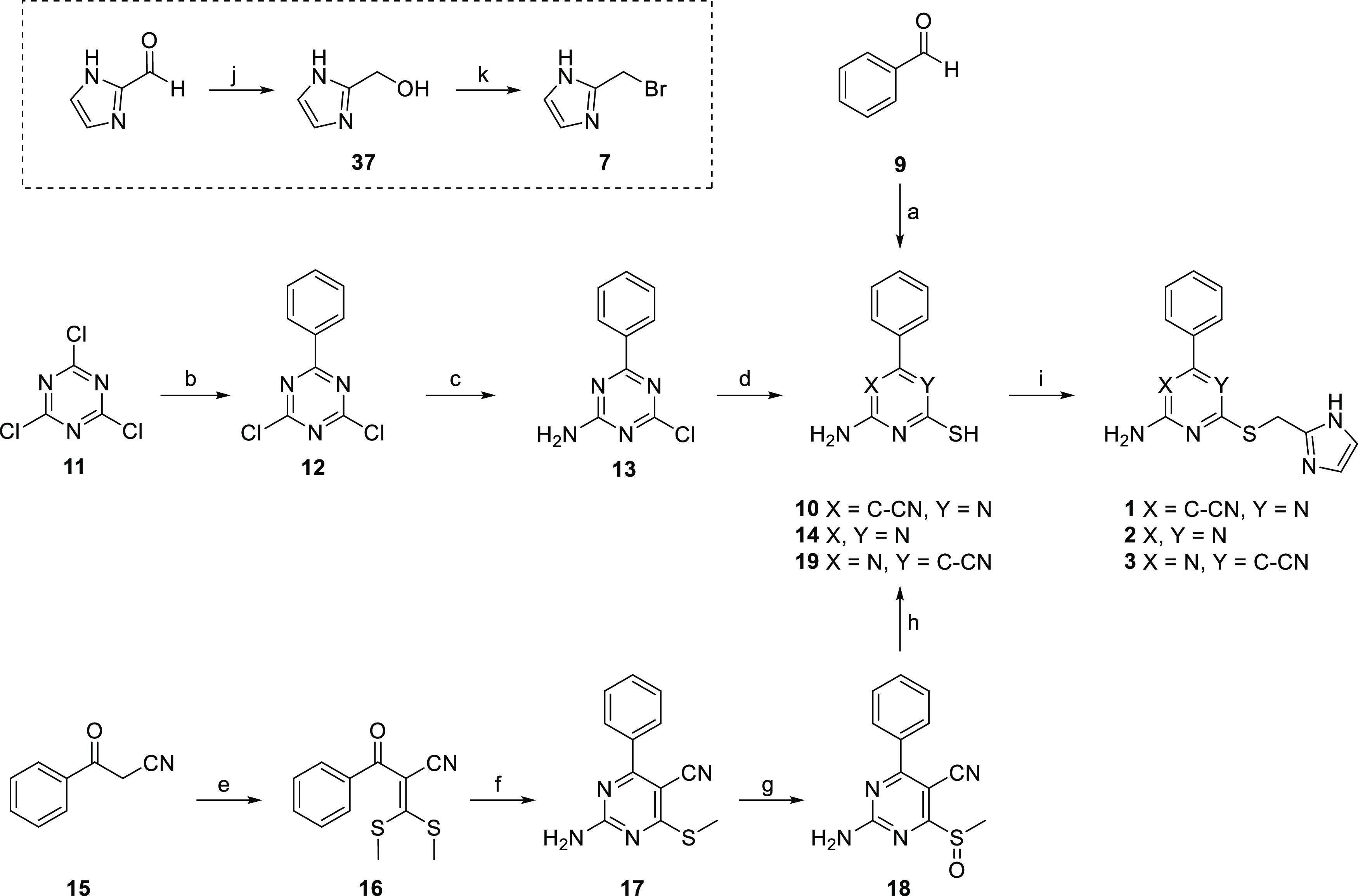
Reagents and conditions: (a) malononitrile, thiourea, K2CO3, EtOH, reflux, 21% (for 10); (b) R-phenylmagnesium bromide, dry THF, N2, rt, 52%; (c) NH3 (25%) in H2O, CH2Cl2, rt, 66%; (d) Na2S·9H2O, DMF, 80 °C, 28% (for 14); (e) NaH, carbon disulfide, iodomethane, dry DMSO, N2, rt, 82%; (f) guanidine hydrochloride, TEA, DMF, reflux, 42%; (g) mCPBA,CH2Cl2, rt, 66%; (h) potassium thioacetate, DMF, rt, 53% (for 19); (i) 7, Na2CO3, DMF, rt, 3–46%; (j) NaBH4, absolute EtOH, rt, 75%; (k) 33% HBr in CH3COOH, reflux, 65%.
