Scheme 2. Synthesis Route toward Pyridine Derivatives 4–6(25).
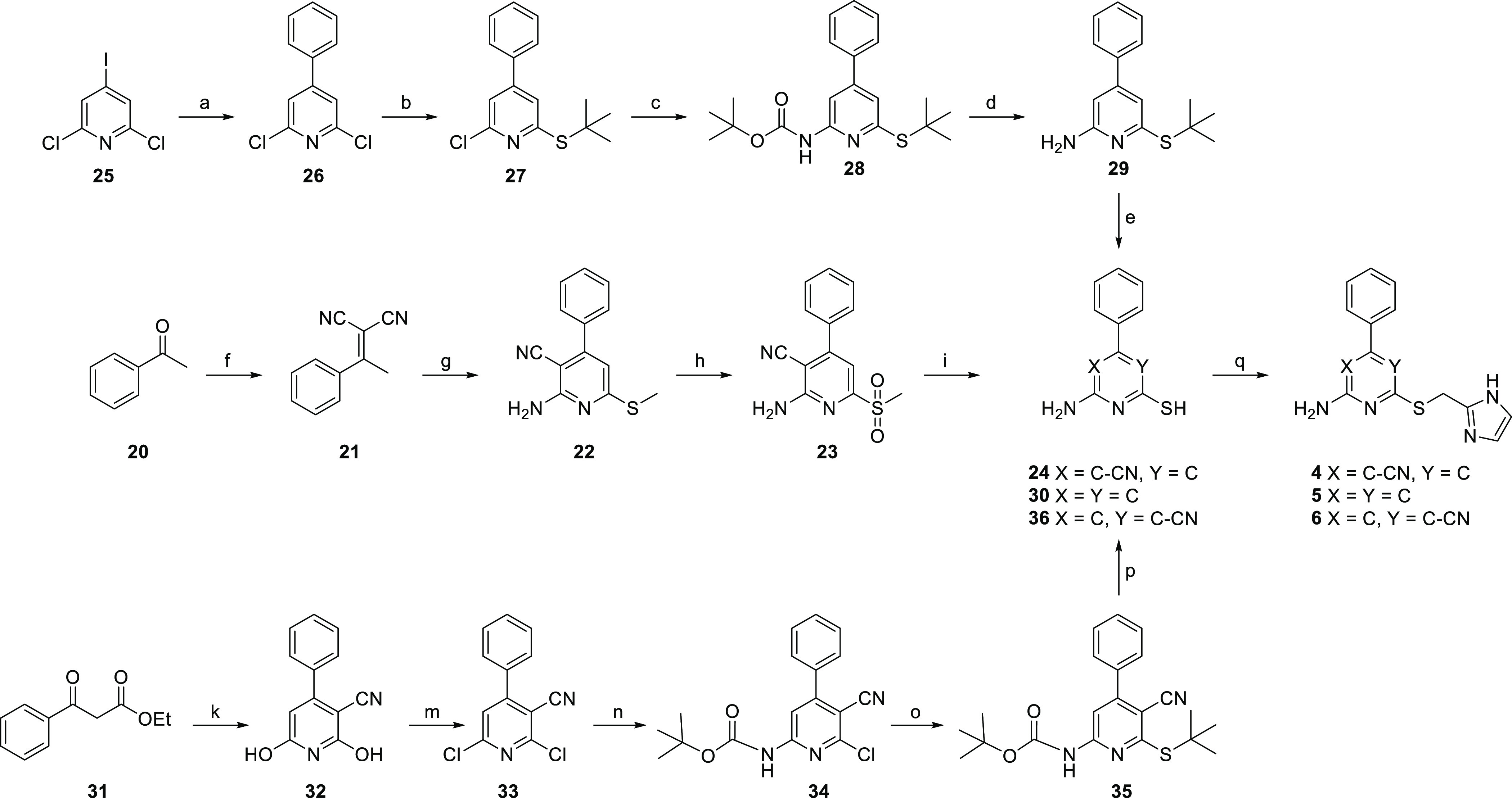
Reagents and conditions: (a) phenylboronic acid pinacol esther, Pd(PPh3)Cl2, Na2CO3, H2O/MeCN, N2, 70 °C, 92%; (b) 2-methyl-propanethiol, Cs2CO3, DMF, 80 °C, quantitative yield; (c) Pd(OAc)2, Xantphos, Cs2CO3, t-butyl carbamate, dry 1,4-dioxane, N2, 110 °C, 27%; (d) TFA, CH2Cl2, reflux, 75%; (e) 37% HCl, 100 °C; (f) malononitrile, ammonium acetate, toluene, reflux in Dean–Stark apparatus, 70%; (g) (i) dimethyl cyanocarbonimidodithioate, K2CO3, DMF, rt, (ii) piperidine, 80 °C, 62%; (h) mCPBA,CH2Cl2, rt, 56%; (i) potassium thioacetate, DMF, rt, 61% (for 24); (k) 2-cyanoacetamide, KOH, EtOH, reflux, 34%; (m) POCl3 in an autoclave, 180 °C, 70%; (n) Pd(OAc)2, Xantphos, Cs2CO3, t-butyl carbamate, dry 1,4-dioxane, N2, 40 °C, 33%; (o) 2-methyl-propanethiol, Cs2CO3, DMF, 90 °C, 89%; (p) 37% HCl, 100 °C, 37% (for 36); (q) 7, NaHCO3, DMF, rt, 8–57%.
