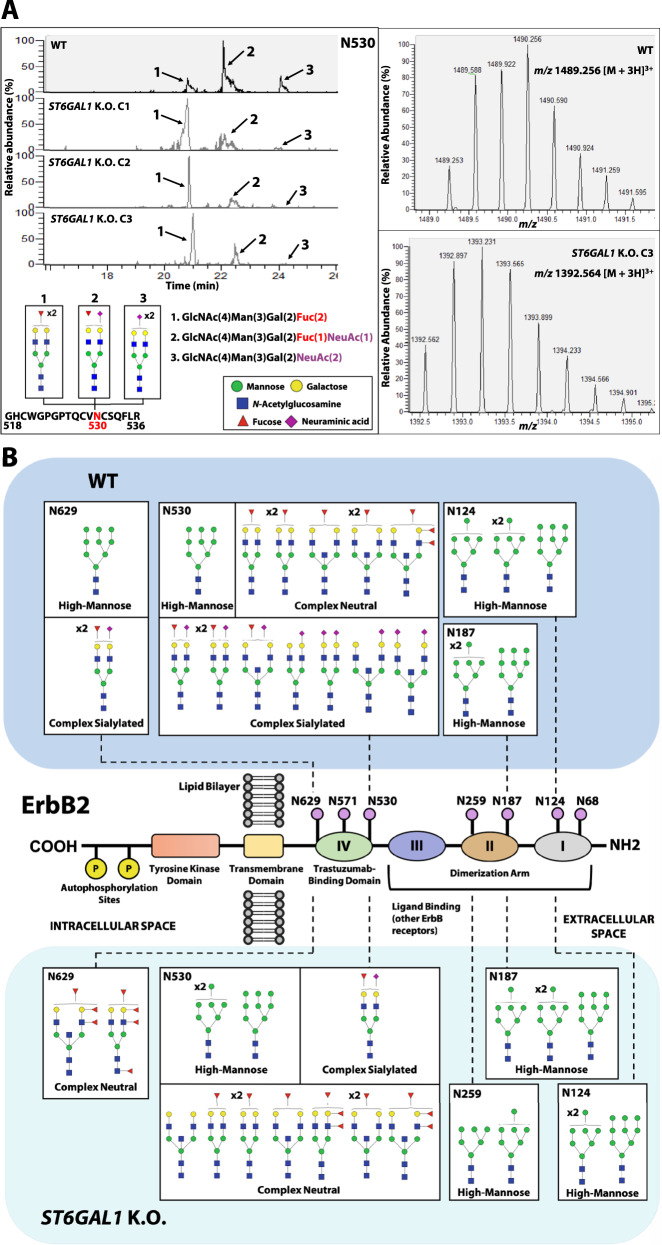
Fig. 5. ST6Gal1 specifically targets glycosylation sites within ErbB2 trastuzumab-binding domain.
A Left panel—combined extracted ion chromatogram of the N530 glycopeptide modified with: a di-fucosylated (dF) biantennary N-glycan (peak 1, peptide + GlcNAc(4)Man(3)Gal(2)Fuc(2), ion at m/z 1392.564, [M + 3H]3+), a mono-sialylated mono-fucosylated (mSmF) biantennary N-glycan (peak 2, peptide + GlcNAc(4)Man(3)Gal(2)Fuc(1)NeuAc(1), ion at m/z 1440.910, [M + 3H]3+) and di-sialylated (dS) biantennary N-glycan biantennary N-glycan (peak 3, peptide + GlcNAc(4)Man(3)Gal(2)NeuAc(2) ion at m/z 1489.256, [M + 3H]3+) in WT and ST6GAL1 K.O. C3 ErbB2. Right panels—isotopic distribution of the N530 glycopeptide modified with a dS biantennary N-glycan in WT ErbB2, and with a dF biantennary N-glycan in ST6GAL1 K.O. C3 ErbB2; B Schematic representation of glycosylation site assignment and structural characterization in WT and ST6GAL1 K.O. ErbB2; C1 clone 1, C2 clone 2, C3 clone 3.