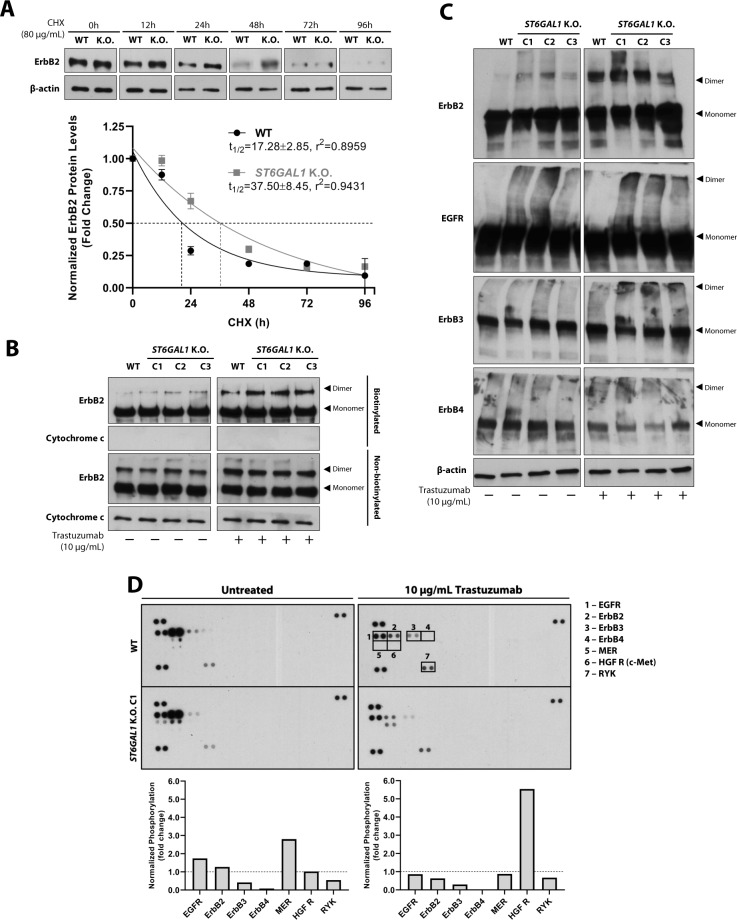
Fig. 7. ST6GAL1 K.O. increases ErbB2 protein half-life and potentiates the trastuzumab-induced stabilization of ErbB dimers at the cell membrane.
A Western blot analysis of ErbB2 in whole cell lysates of NCI-N87 WT and ST6GAL1 K.O. C1 cells after blocking protein synthesis with 80 μg/mL cycloheximide (CHX) for 0, 12, 24, 48, 72, and 96 h. ErbB2 protein half-life was calculated based on the density of the β-actin-normalized ErbB2 Western blot bands, using the one phase exponential decay function, and was defined as the time required for the ErbB2 protein to reach 50% of its initial level; n = 3 (mean ± SD); B Western blot analysis of biotinylated membrane-bound and non-biotinylated cytoplasmic ErbB2 from NCI-N87 WT and ST6GAL1 K.O. cells treated with 10 μg/mL trastuzumab for 24 h. The mitochondrial marker cytochrome c was used as a control for non-biotinylated proteins from the cytoplasmic subcellular compartment; C Western blot analysis of ErbB2, EGFR, ErbB3, and ErbB4 dimerization in whole cell lysates of NCI-N87 WT and ST6GAL1 K.O. cells cross-linked with bis(sulfosuccinimidyl)suberate (BS3), following treatment with 10 μg/mL trastuzumab for 24 h; D Analysis and quantification of the phosphorylation levels of 49 cancer-related cell membrane receptor tyrosine kinases (RTKs) in NCI-N87 WT and ST6GAL1 K.O. cells treated with 10 μg/mL trastuzumab for 120 h.