Figure 2. Txnip Cys247 regulates the induction of oxidative stress and cell death under hypoxic conditions.
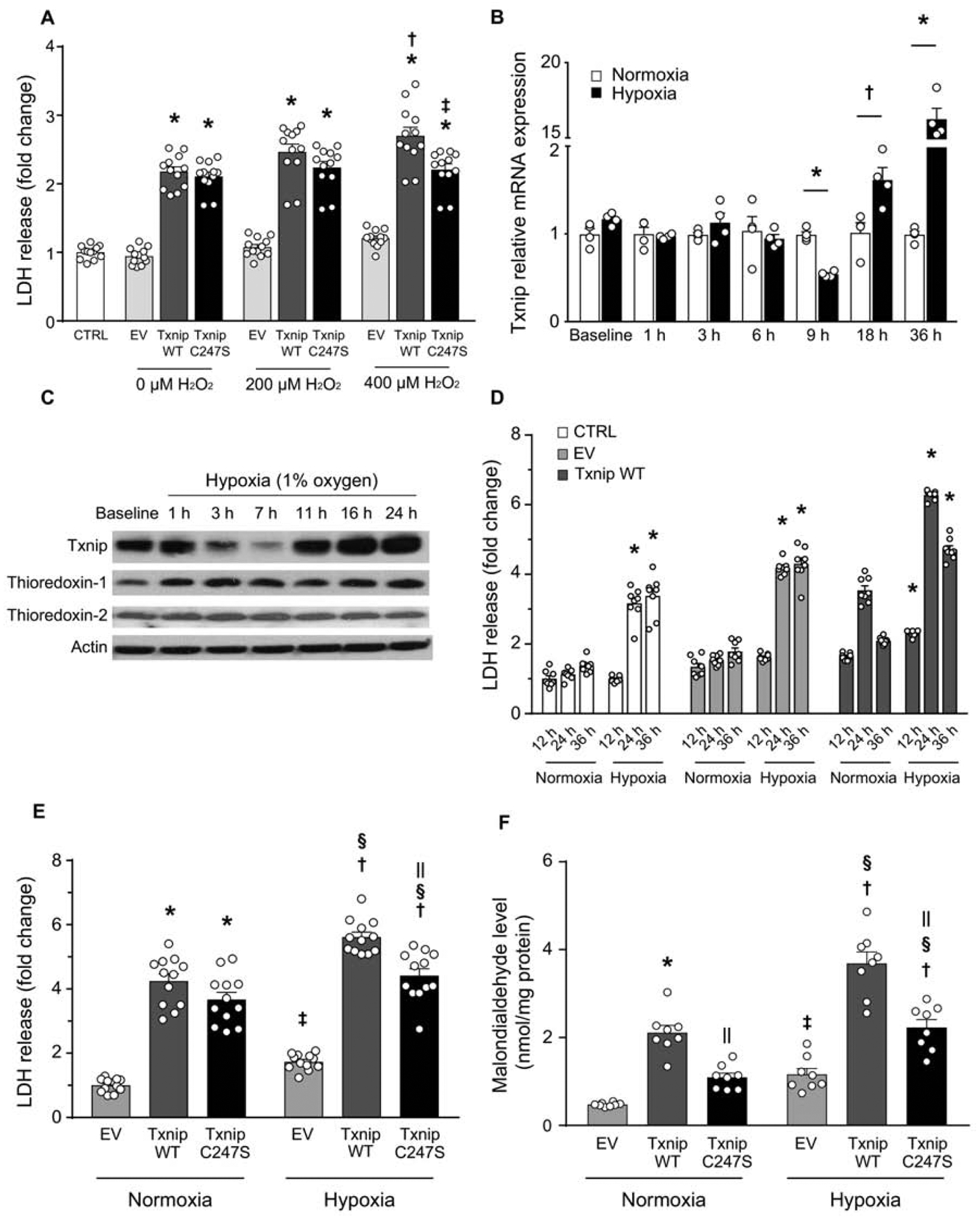
A. Txnip C247S blocked ROS-induced cell death. MEFs derived from Txnip-knockout mice were reconstituted with adenoviral gene transfer of Txnip wild-type (WT), C247S, or empty vector (EV). Cells were incubated with indicated concentrations of hydrogen peroxide (H2O2) for 3 hrs. *P<0.01 EV, †P<0.01 vs. 0 μM Txnip WT, ‡P<0.01 vs. 400 μM Txnip WT. B. Time-dependent changes of Txnip mRNA expression by hypoxia (1% O2) in SKBR3 cells. *P<0.01 and †P<0.05 vs. normoxia. C. Western blot analyses in H9c2 rat cardiomyocytes. Txnip expressions were sensitive to oxygen levels. D. Adenoviral overexpression of Txnip enhanced lactate dehydrogenase (LDH) release from SKBR3 cells under hypoxia. *P<0.01 vs. normoxia. E and F. Txnip-null MEFs were transfected with adenoviruses encoding Txnip WT, C247S, or EV. Hypoxia (1% O2, 24 hrs) increased LDH release and cellular production of malondialdehyde, a marker of oxidative stress. C247S reduced hypoxia-induced cellular damage and formation of ROS. *P< 0.01 vs. EV normoxia, †P<0.01 vs. EV hypoxia, ‡P<0.05 vs. EV normoxia, §P<0.01 vs. the same adenovirus under normoxia, and ||P<0.01 vs. Txnip WT under the same condition.
