Figure 3. The Cre-loxP recombination strategy for conditional cardiomyocyte-specific knock-in mutation of Txnip.
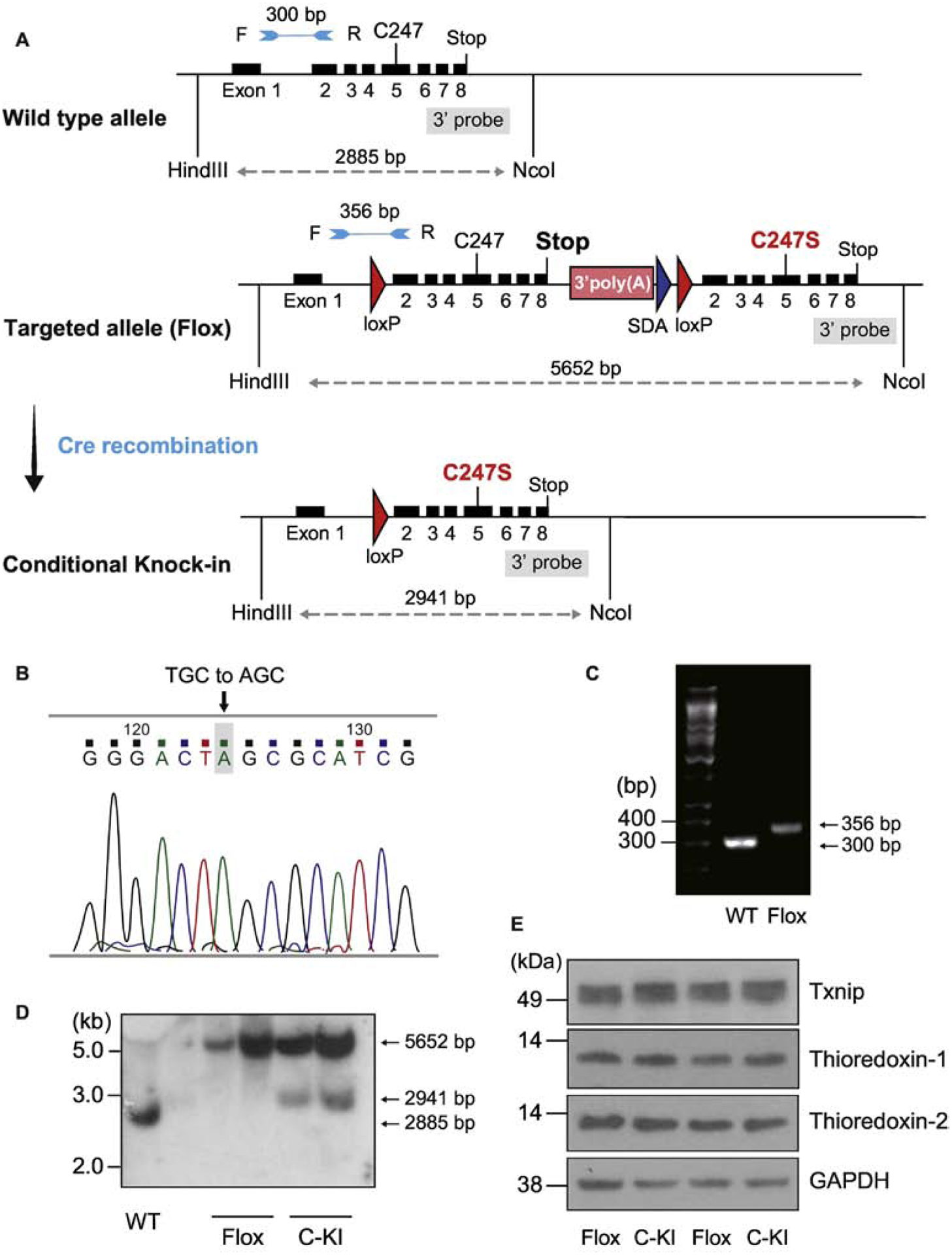
A. Two loxP sites were introduced in the targeting construct for Cre-mediated deletion of exons 2–8. Another set of exons 2–8 containing C247S mutation in exon5 was incorporated after the second loxP site. By breeding floxed mice with MerCreMer expressing mice, deletion of the floxed site was achieved by administration of tamoxifen, which resulted in the temporally regulated knock-in mutation of C247S under the control of wild-type (WT) gene regulatory element. B. Successful single mutation was confirmed by DNA sequencing in the mouse. C. WT and homozygous Txnip knock-in mice were characterized by PCR with forward (F) and reverse (R) primers, allowing the amplification of the loxP-containing deletion region; a 356-bp fragment for flox and a 300-bp fragment for WT allele. D. Successful recombination events were confirmed by Southern blot analyses. HindIII and NcoI-digested DNA was hybridized with the 3’ probe (5652-bp, 2911-bp, and 2885-bp bands represent the flox, conditional knock-in (C-KI), and WT allele, respectively). E. After the recombination, protein expression levels of Txnip, thioredoxin-1, and thioredoxin-2 were comparable in mouse hearts between flox mice and cardiomyocyte-specific knock-in mice.
