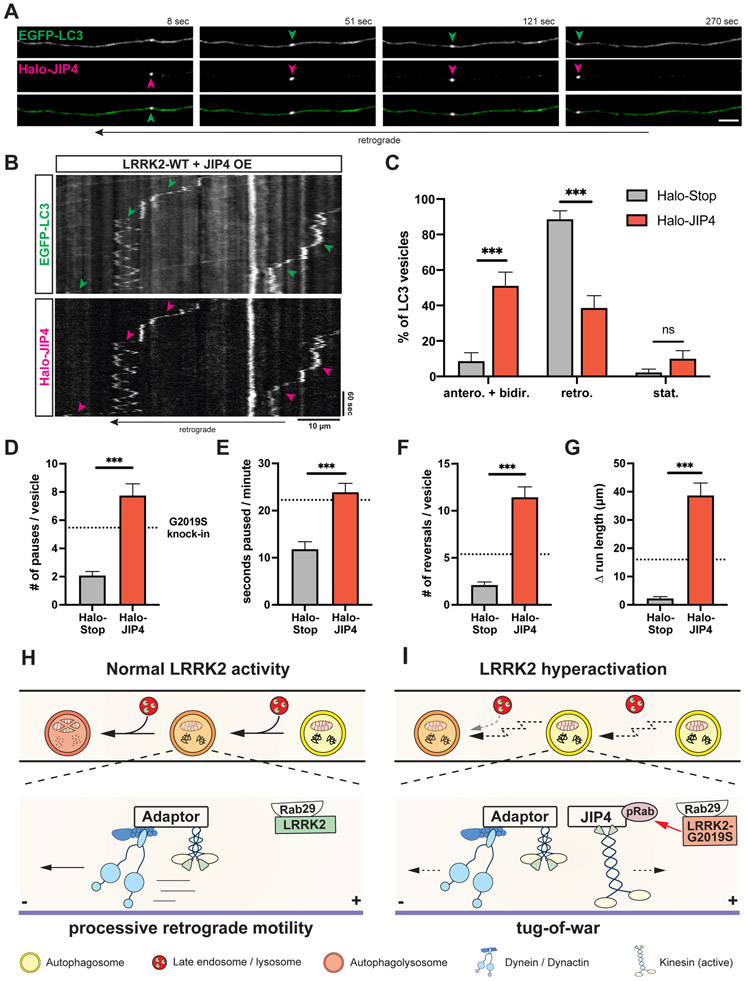
Figure 7. Overexpression of JIP4 disrupts AV transport.
(A) Time lapse images of axonal EGFP-LC3 and Halo-JIP4 vesicles in a mouse WT cortical neuron. Scale bar, 5 μm. See also Video S7. (B) Kymographs of axonal EGFP-LC3 and Halo-JIP4 vesicles. Arrowheads highlight comigration. (C) Directionality of EGFP-LC3 vesicles in Halo-Tag only and Halo-JIP4 overexpressing WT neurons. Antero + bidir., anterograde + bidirectional; retro., retrograde; stat., stationary (mean ± SEM; n = 24-25 neurons from 3 independent cultures; ***p<0.0001; Two-way ANOVA). (D) Pause number, (E) Fraction of time paused, (F) Number of reversals, (G) Δ run length of AVs in Halo-Tag only and Halo-JIP4 overexpressing neurons (mean ± SEM; n = 68-87 AVs from 24-25 neurons from 3 independent cultures; ***p<0.0001; Mann-Whitney test). Dotted lines indicate the respective average observed in mouse G2019S KI neurons. (H-I) Model depicting the effect of increased LRRK2 kinase activity on axonal AV transport and maturation. (H) Normal LRRK2 activity allows for processive retrograde AV transport, facilitating efficient fusion en route with lysosomal vesicles. Motor adaptors inhibit kinesin and promote dynein activity, resulting in processive retrograde transport. (I) LRRK2 hyperactivation by LRRK2-G2019S mutation or Rab29 overexpression disrupts processive AV transport, leading to inefficient autophagosome-lysosome fusion and impaired AV acidification. Hyperactive LRRK2 enhances recruitment of JIP4 to the AV membrane via binding to phospho-Rabs, resulting in abnormal kinesin activation and a tug-of-war between anterograde and retrograde motors.