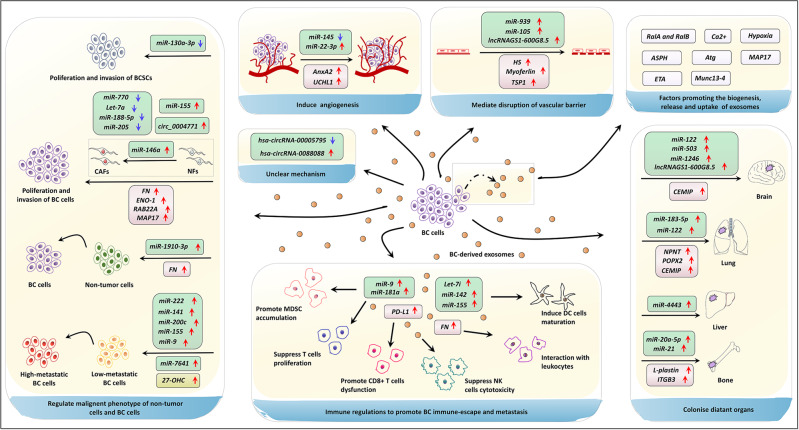
Fig. 3. The multifaceted roles and mechanisms of tumor-derived exosomal components in breast cancer metastasis.
Exosomes could mediate metastatic initiation and progression of BC by transferring functional cargos. BC-derived exosomes can modify the malignant phenotype of non-tumor cells and low-metastatic BC cells such as promoting proliferation and invasion of BCSCs, promoting proliferation and invasion of BC cells through the transformation of NFs to CAFs. Besides, the specific cargos are also involved in metastasis via inducing angiogenesis and disruption of vascular permeability. Furthermore, tumor-derived exosomes participate in organotypic metastasis. Through lymphatic circulation and blood circulation, primary BC-derived exosomes can transfer proteins (e.g., NPNT) and nucleic acids (e.g., miRNAs) to distant organs, thus inducing colonization and infiltration of new sites. The key procedure for establishing a permissive environment for BC metastasis is also related to interaction between BC cells and immune cells to escape immune surveillance. For example, BC exosomes carried PD-L1 could promote CD8+ T-cell dysfunction to facilitate immune-suppressing and metastasis. Most studies have confirmed the expression levels and metastasis capabilities of cargos in exosomes involving in BC metastasis, while a few studies only confimed the genetic changes of cargoes by sequencing. Finally, the role of exosomes in BC metastasis is also associated with factors affecting exosomal biogenesis, release and uptake. For example, hypoxia and ETA can promote BC metastasis by promoting the release of exosomes. ↑ upregulation, ↓ downregulation, breast cancer BC, human breast cancer stem cells BCSCs, normal fibroblasts NFs, cancer-associated fibroblasts CAFs, interleukin IL, tumor necrosis factor TNF, microRNA miRNA, long noncoding RNA lncRNA, circular RNA circRNA, extracellular matrix protein nephronectin NPNT, cell migration-inducing and hyaluronan-binding protein CEMIP, ubiquitin carboxyl-terminal hydrolase isozyme L1 UCHL1, integrin β3 ITGB3, 27-hydroxycholesterol 27-OHC, thrombospondin-1 TSP1, fibroblast growth factor FGF, platelet-derived growth factor PDGF, annexin A2 AnxA2, enolase-1 ENO-1, heparan sulfate HS, autophagy and autophagy-related genes Atg, endothelin receptor A ETA, asparaginyl β-hydroxylase ASPH, myeloid-derived suppressor cells MDSC, dendritic cells DC, and programmed death-ligand 1 PD-L1.