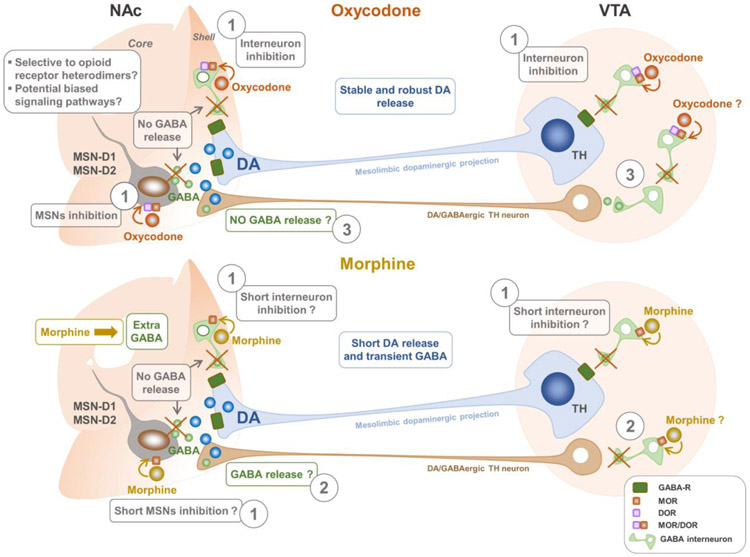
Fig. 2. Action of oxycodone and morphine on potential GABAergic neurons responsible for the difference in the extracellular dopamine (DA) within the reward system.
Oxycodone and morphine provoke distinct changes in DA release in the nucleus accumbens (NAc) that could potentially explain drug users’ selective preference for oxycodone over other opioids (Vander Weele et al. 2014). Intravenous infusion of oxycodone leads to greater global and phasic releases of DA in the NAc (especially in the shell), while morphine causes a short-lived DA release coupled to transient GABA release within the same structure (Vander Weele et al. 2014). Dopaminergic neurons of the mesolimbic pathway project from the VTA in the midbrain onto the GABAergic inhibitory medium spiny neurons (MSNs) in the NAc (Malenka et al. 2009; Ikemoto et al. 2010; Yager et al. 2015; Morales and Margolis 2017). These MSNs represent 95% of the striatum and express the D1 and/or D2 dopaminergic receptors (Kemp and Powell 1971; Yager et al. 2015; Morales and Margolis 2017). Besides DA, GABA is one of the principal neurotransmitters that mediate reward signaling within the NAc (Meredith et al. 1992; Shirayama et al. 2006). Terminals of dopaminergic neurons in the NAc from the VTA are presumed to express GABA receptors through which GABA released from the MSNs may induce tonic inhibition (Yang et al. 2018). MOR has been shown to be expressed on MSNs (Svingos et al. 1999; Shippenberg et al. 2008) and in an undetermined subset of GABAergic neurons within the mouse NAc (Ford et al. 2006; Margolis et al. 2012, 2014; Hinkle et al. 2019). Several lines of indirect functional evidence support the presence of MOR on GABAergic neurons in the VTA (Margolis et al. 2014). As posits the canonical model, opioids induce reward by disinhibiting VTA and NAc dopaminergic neurons through inhibition of GABAergic inputs (Johnson and North 1992; Labouèbe et al. 2007; Barrot et al. 2012; Bourdy and Barrot 2012; Margolis et al. 2012). Therefore, oxycodone may cause robust and stable DA release by decreasing the excitability of GABAergic MSNs (see label 1 on the figure), thus blocking their potential tonic inhibitory action on the DA terminals in the NAc. Oxycodone may also inhibit other NAc interneurons that innervate directly the dopaminergic terminals (label 1) and the GABAergic inputs on these dopaminergic neurons in the VTA (label 1). As our unpublished data suggest, the robust and stable increase in global and phasic releases of DA in the presence of oxycodone might involve the selective stimulation of putative opioid receptor heterodimers or potentially different signaling cascades compared to morphine. The morphine-dependent short-lived DA response could be caused by the additional surge of GABA release following morphine. One hypothesis for the transient rise of GABA is that inhibition of GABAergic neurons by morphine could be short-lived compared to oxycodone, thus leading to a brief inhibition of GABA release (bottom figure). However, this needs to be demonstrated. The surplus of GABA could also be secreted by VTA DA/GABAergic TH-expressing neurons that project to the NAc (labels 2 and 3). We speculate that these latter neurons could be activated to release GABA via disinhibition by morphine (label 2), whereas they would be inhibited in the presence of oxycodone via a potential selective agonist action on opioid receptor heterodimers-expressing GABAergic neurons (label 3).