Table 1.
Recommended belamaf dose modifications based on eye examination findings per the KVA scale per the US prescribing information and the EU summary of product characteristics combined22,23.
| Severitya | Corneal examination findingsb | Change in BCVA due to treatment-related corneal findings | Recommended dose modifications | ||
|---|---|---|---|---|---|
| Description | Presentation of MECs (based on density and location) | Example schematics of MECs by severity | |||
| Grade 1/Mild | Mild superficial keratopathyc (documented worsening from baseline) with or without symptoms |
Mild density: non-confluent Location: predominantly (≥80%) peripheral |
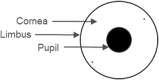 |
Decline from baseline of 1 line on Snellen Visual Acuity | Continue treatment at current dose |
| Grade 2/Moderate | Moderate superficial keratopathyc with or without patchy microcyst-like deposits, sub-epithelial haze (peripheral), or a new peripheral stromal opacity |
Moderate density: semi-confluent Location: predominantly (≥80%) paracentral |
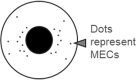 |
Decline from baseline by 2 or 3 lines (and Snellen Visual Acuity not worse than 20/200) |
Withhold treatment until improvement in either exam findings or BCVA to Grade 1/mild • Resume at a reduced dose of 1.9 mg/kg |
| Grade 3/Severe | Severe superficial keratopathyc with or without diffuse microcyst-like deposits involving the central cornea, sub-epithelial haze (central), or a new central stromal opacity |
Severe density: confluent Location: predominantly (≥80%) central |
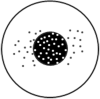 |
Decline from baseline of more than 3 lines (and Snellen Visual Acuity not worse than 20/200) |
Withhold treatment until improvement in either exam findings or BCVA to Grade 1/mild • Resume at a reduced dose of 1.9 mg/kgd |
| Grade 4/Severe | Corneal epithelial defect, including corneal ulcers. These should be managed promptly and as clinically indicated by an eye care professional | Not applicable, these events are not graded based on MECs | Snellen Visual Acuity worse than 20/200 |
Withhold treatment until improvement in examination findings and BCVA to Grade 1/mild. Consider treatment discontinuation based on a benefit–risk assessment. For worsening symptoms that are unresponsive to appropriate management, consider discontinuation • If continuing treatment, resume at a reduced dose of 1.9 mg/kgd |
|
BCVA best-corrected visual acuity, belamaf belantamab mafodotin, KVA keratopathy and visual acuity, MEC microcystic-like epithelial changes.
aThe severity category is defined by the more severely affected eye as both eyes may not be affected to the same degree. Prescribing physicians should refer to the guidelines for corneal adverse event management in their local labeling.
bThe worst severity for MEC density or location should be used in grading. Grading is based on the worst finding in the worse-affected eye. These evaluations and examples do not apply to, or include, superficial punctate keratopathy.
cPatients may have superficial punctate keratopathy, MECs, or both. Keratopathy refers to superficial punctate keratopathy (revealed by fluorescein staining) or MECs (which may not stain with fluorescein). Fluorescein staining should be part of each eye examination, including the baseline examination. The worst grade for the keratopathy and the change in BCVA should be used to determine the grade of the corneal adverse event.
dThe restarting dose for Grade 3/4 events per the US prescribing information.
