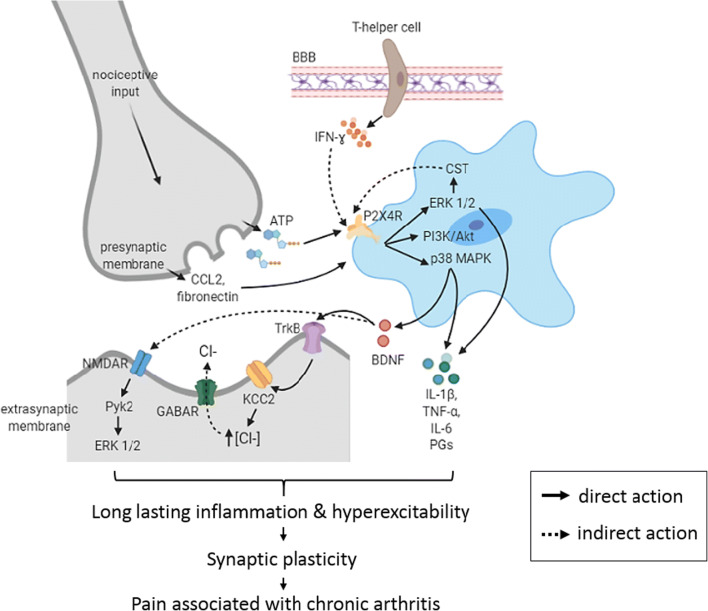
Fig. 3.
Central mechanisms after P2X4R activation in the spinal cord. The affected neuron secretes adenosine triphosphate (ATP), chemokine ligand 2 (CCL2) and fibronectin that upregulate microglia P2X4 receptor (P2X4R) and promote its trafficking to cell surface prior to peripheral nerve injury due to rheumatoid arthritis (RA). Helper T-cells from peripheral blood vessel may pass through the blood-brain barrier (BBB) and interact with microglia via the released pro-inflammatory cytokines including interferon-ɣ (IFN-ɣ). As a consequence of ATP binding to P2X4R, several pathways are activated including phosphorylation of extracellular signal-regulated kinase 1/2 (ERK 1/2) and p38 mitogen-activated protein kinase (p38 MAPK) and phosphatidylinositol 3-kinases-protein kinase B (PI3K/Akt) that leads to the release of brain-derived neurotrophic factor (BDNF) and pro-inflammatory mediators (IL-1β, TNF-α, IL-6 and prostaglandins) that contributes to neurogenic inflammation. Secreted BDNF may then bind to tropomyosin receptor kinase B (TrkB) receptors on nociceptive neurons that further downregulate potassium chloride cotransporter (KCC2) resulting in the accumulation of chloride anions (Cl-) in the neurons. Consequently, the subsequent binding to ɣ-aminobutyric acid A receptor (GABAA receptor) that is normally inhibitory, shifts to the disinhibition of inhibitory neurons. Meanwhile, the production of cathestatin (CST) via the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK 1/2) in microglia may further augment the P2X4R activation on the activated microglia. The hyperexcitability resulted from GABA depolarisation together with the release of pro-inflammatory cytokines from microglia and lesioned nerve, as well as N-methyl-d-aspartate receptors (NMDARs) activation, leads to sensitisation of spinal circuits and therefore, chronic pain