Table 1. In Vitro Activity of 2,4-Disubstituted 5-Nitro-6-thiocyanatopyrimidines 63–112 against Cryptococcus neoformans and Cryptococcus gattii.
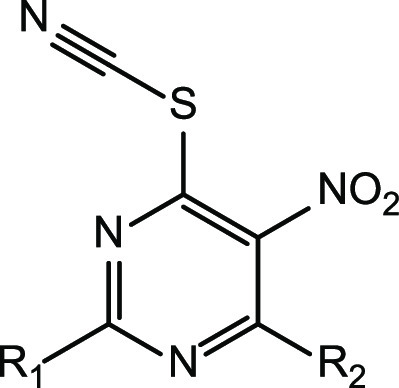
| molecule no. | R1 | R2 | CC50 μM (μg/mL) | C. neoformans MIC80 μM (μg/mL) | C. gattia MIC80 μM (μg/mL) | C. neoformansa MIC80 (FLC-resistance) μM (μg/mL) |
|---|---|---|---|---|---|---|
| 63 | H | MeO | 9.37 ± 3.12 (1.98 ± 0.66) | >25 (>5.3) | ||
| 64 | H | PrO | 31.8 (7.64) | 3.9 ± 0 (0.93 ± 0) | 4.68 ± 1.56 (1.12 ± 0.38) | |
| 65 | H | iPrO | 23.3 (5.6) | 1.9 ± 0 (0.45 ± 0) | 1.17 ± 0.39 (0.27 ± 0.09) | |
| 66 | H | NH2 | 4.68 ± 1.56 (0.92 ± 0.3) | 1.17 ± 0.39 (0.22 ± 0.07) | ||
| 67 | H | iPrNH | 12 (2.87) | 2.34 ± 0.78 (0.55 ± 0.18) | 0.58 ± 0.19 (0.13 ± 0.04) | 2.34 ± 1.56 (0.55 ± 0.37) |
| 68 | H | 3-methylbutan-2-yl-NH | 3 ± 0 (0.8 ± 0) | |||
| 69 | H | pentan-3-yl-NH | 18.5 ± 0 (4.94 ± 0) | >25 (>6.678) | ||
| 70 | H | cyclopentyl-NH | 4.9 ± 0 (1.29 ± 0) | 1.17 ± 0.39 (0.3 ± 0.1) | 2.34 ± 1.56 (0.62 ± 0.41) | |
| 71 | H | cyclohexyl-NH | 6 ± 0 (1.67 ± 0) | 1.17 ± 0.39 (0.32 ± 0.11) | ||
| 72 | H | piperidinyl | 11.9 (3.16) | 2.34 ± 0.78 (0.61 ± 0.2) | 1.17 ± 0.39 (0.3 ± 0.1) | 4.68 ± 3.13 (1.24 ± 0.83) |
| 73 | H | 2-Me-piperidinyl | 9.37 ± 3.12 (2.61 ± 0.86) | 1.17 ± 0.39 (0.32 ± 0.11) | ||
| 74 | H | 2,6-di-Me-piperidinyl | 9.37 ± 3.12 (2.74 ± 0.9) | 15.62 ± 9.37 (4.58 ± 2.75) | ||
| 75 | H | 3,5-di-Me-piperidinyl | 33.6 (9.86) | 2.34 ± 0.78 (0.68 ± 0.22) | 9.37 ± 3.12 (2.74 ± 0.91) | |
| 76 | H | azepanyl | 17.7 (4.94) | 2.34 ± 0.78 (0.65 ± 0.22) | 2.34 ± 0.78 (0.65 ± 0.22) | 2.34 ± 1.56 (0.65 ± 0.43) |
| 77 | H | azocanyl | 4.68 ± 1.56 (1.37 ± 0.46) | 2.34 ± 0.78 (0.68 ± 0.23) | ||
| 78 | H | benzyl-NH | 19.6 (5.63) | 2.34 ± 0.78 (0.66 ± 0.22) | 1.17 ± 0.39 (0.33 ± 0.11) | 1.17 ± 0.78 (0.33 ± 0.22) |
| 79 | H | (mCF3-benzyl)-NH | 25.91 (9.21) | 9.37 ± 12.5 (3.32 ± 4.44) | 4.68 ± 1.56 (1.66 ± 0.56) | |
| 80 | H | (pCl-benzyl)-NH | 22.53 (7.25) | 5.85 ± 7.8 (1.88 ± 2.5) | 1.17 ± 0.39 (0.37 ± 0.12) | |
| 81 | H | Ph-(CH2)2-NH | 15.09 (4.55) | 3.51 ± 4.68 (1.05 ± 1.4) | 2.34 ± 0.78 (0.7 ± 0.23) | |
| 82 | H | Me2N | 4.68 ± 1.56 (1.05 ± 0.34) | 1.17 ± 0.39 (0.26 ± 0.09) | ||
| 83 | H | di-Et-N | 9.37 ± 3.12 (2.37 ± 0.78) | 0.14 ± 0.04 (0.03 ± 0.01) | ||
| 84 | H | iPrEtN | 10.7 (2.86) | 4.55 ± 4.24 (1.4 ± 1.12) | 1.17 ± 0.39 (0.3 ± 0.1) | |
| 85 | H | cyclohexylMeN | 25.3 (7.42) | 6 ± 0 (1.76 ± 0) | 4.68 ± 1.56 (1.37 ± 0.46) | |
| 86 | H | benzylMeN | 18.8 (5.66) | 2.34 ± 0.78 (0.7 ± 0.22) | 1.17 ± 0.39 (0.35 ± 0.12) | |
| 87 | H | benzyl-N-iPr | >50 (>16.46) | >25 (>8.23) | ||
| 88 | Me | EtO | 19.8 (4.76) | 1.17 ± 0.38 (0.27 ± 0.08) | 0.29 ± 0.1 (0.06 ± 0.02) | 2.34 ± 1.56 (0.56 ± 0.37) |
| 89 | Me | PrO | 15.8 (4.02) | 0.6 ± 0 (0.15 ± 0) | 1.17 ± 0.39 (0.29 ± 0.1) | 1.17 ± 0.78 (0.29 ± 0.19) |
| 90 | Me | iPrO | 14.6 (3.71) | 1 ± 0 (0.25 ± 0) | 1.17 ± 0.39 (0.29 ± 0.1) | 1.17 ± 0.78 (0.29 ± 0.19) |
| 91 | Me | NH2 | 24.5 (5.17) | 2.34 ± 0.78 (0.48 ± 0.16) | 0.58 ± 0.19 (0.12 ± 0.04) | 2.34 ± 1.56 (0.49 ± 0.32) |
| 92 | Me | MeNH | >100 (>22.53) | 2.34 ± 0.78 (0.52 ± 0.16) | 1.17 ± 0.39 (0.26 ± 0.09) | 2.34 ± 1.56 (0.52 ± 0.35) |
| 93 | Me | pentan-3-yl-NH | 9 ± 0 (2.53 ± 0) | 9.37 ± 3.12 (2.63 ± 0.88) | ||
| 94 | Me | cyclopropyl-NH | 12.7 (3.19) | 0.6 ± 0 (0.15 ± 0) | 0.58 ± 0.19 (0.14 ± 0.05) | 1.17 ± 0.78 (0.29 ± 0.19) |
| 95 | Me | cyclopentyl-NH | 9 ± 0 (2.51 ± 0) | 2.34 ± 0.78 (0.65 ± 0.22) | ||
| 96 | Me | cyclohexyl-NH | >50 (>24.62) | >25 (>12.31) | ||
| 97 | Me | pyrrolidinyl | 4.68 ± 1.56 (1.23 ± 0.4) | 1.17 ± 0.39 (0.3 ± 0.1) | ||
| 98 | Me | 2-Me-piperidinyl | 18.75 ± 6.24 (5.49 ± 1.82) | 0.58 ± 0.19 (0.16 ± 0.05) | ||
| 99 | Me | 3,5-di-Me-piperidinyl | 18.75 ± 6.24 (5.76 ± 1.92) | 9.37 ± 3.12 (2.88 ± 0.96) | ||
| 100 | Me | azepanyl | 18.75 ± 6.24 (5.49 ± 1.82) | 4.68 ± 1.56 (1.37 ± 0.46) | ||
| 101 | Me | azocanyl | 18.75 ± 6.24 (5.76 ± 1.92) | 2.34 ± 0.78 (0.71 ± 0.24) | ||
| 102 | Me | benzyl-NH | 15.87 (4.78) | 50 ± 0 (15.06 ± 0) | 18.75 ± 6.25 (5.64 ± 1.88) | |
| 103 | Me | (pMe-benzyl)-NH | 14.73 (4.65) | 50 ± 0 (15.76 ± 0) | 1.17 ± 0.39 (0.36 ± 0.12) | |
| 104 | Me | Ph-(CH2)2-NH | 12.27 (3.87) | 50 ± 0 (15.76 ± 0) | 2.34 ± 0.78 (0.73 ± 0.24) | |
| 105 | Me | Me2N | 4.68 ± 1.56 (1.11 ± 0.36) | 2.34 ± 0.78 (0.55 ± 0.18) | ||
| 106 | Me | di-Et-N | 9.37 ± 3.12 (2.5 ± 0.82) | 2.34 ± 0.78 (0.62 ± 0.21) | ||
| 107 | SMe | benzyl-NH | 18.59 (6.2) | 4.68 ± 4.68 (1.56 ± 1.56) | 4.68 ± 1.56 (1.56 ± 0.52) | |
| 108 | SMe | (pMe-benzyl)-NH | 19.2 (6.67) | 9.37 ± 3.12 (3.25 ± 1.08) | 4.68 ± 1.56 (1.62 ± 0.54) | |
| 109 | SMe | Ph-(CH2)2-NH | 15.39 (5.35) | 9.37 ± 9.36 (3.25 ± 3.24) | 1.17 ± 0.39 (0.4 ± 0.13) | |
| 110 | CF3 | iPrNH | 42.8 (13.15) | 12.25 ± 6.5 (4.4 ± 2) | >25 (>7.68) | 18.75 ± 12.5 (5.76 ± 3.84) |
| 111 | Ph | iPrNH | 50 ± 0 (15.76 ± 0) | |||
| 112 | styryl | MeNH | >50 (>15.66) | >25 (>7.83) |
The C. neoformans fluconazole-resistant DUMC-158.03, and C. gattii, RSA-3615, clinical strains were obtained from Dr. John Perfect, Duke University.40
