Abstract
Human cytomegalovirus and Epstein–Barr virus have been recognized as potential drivers of morbidity and mortality of patients undergoing allogeneic stem cell transplantation for years. Specific protocols for monitoring, prophylaxis and pre-emptive therapy are in place in many transplant settings. In this review, we focus on the next three most frequent viruses, human herpesvirus-6, BK virus and adenovirus, causing reactivation and/or viremia after allogeneic transplant, which are increasingly detected in patients in the post-transplant period owing to emerging techniques of molecular biology, recipients’ characteristics, treatment modalities used for conditioning and factors related donors or stem cell source. Given the less frequent detection of an illness related to these viruses, there are often no specific protocols in place for the management of affected patients. While some patients develop significant morbidity (generally older), others may not need therapy at all (generally younger or children). Furthermore, some of the antiviral therapies used are potentially toxic. With the addition of increased risk of secondary infections, risk of graft failure or increased risk of graft-versus-host disease as well as the relationship with other post-transplant complications, the outcomes of patients with these viremias remain unsatisfactory and even long-term survivors experience increased morbidity.
Keywords: allogeneic, posttransplant, HHV-6, BK virus, adenovirus
Human herpesvirus 6 (HHV-6)
Overview
Human herpesvirus 6 (HHV-6) is a relatively large double-stranded DNA virus in the β herpesvirus family that primarily targets CD4+ T lymphocytes, and other cells including monocytes, macrophages, epithelial cells, fibroblastic cells, astrocytes, oligodendrocytes, neurons and hepatocytes.1–3 Two distinct variants of HHV-6 have been identified so far; like other herpesviruses, both variants establish life-long latency and can become reactivated later in life.4 Little is known about the disease history of HHV-6A at this time and no disease causally has been linked to it. In contrast, HHV-6B almost ubiquitously infects all humans in the first 2 years of life. The primary infection may manifest as exanthema subitem (roseola), a febrile rash. Subsequently, HHV-6B reactivation can occur, especially in patients who are immunocompromised. HHV-6 reactivation has been observed in 30–70% of allogeneic hematopoietic stem cell transplantation (alloHSCT) recipients.5,6 It can be asymptomatic, but also has been associated with various disease processes2,7 and potentially worse transplant outcomes,5,8 among which, HHV-6 encephalitis is the most recognized with a well-established cause association.
Unlike other human herpesviruses, HHV-6 has a unique ability to integrate into a chromosomal telomere and transmit through Mendelian inheritance, resulting in virus DNA in every nucleated cell in the body.3 It is estimated that approximately 1% of the population carries chromosomally integrated HHV-6 (ciHHV-6)9 with one-third being ciHHV-6A and two-thirds being ciHHV-6B.10 It is important to note that in patients with ciHHV-6, who can be either transplant recipients or donors, viral DNA is persistently detected in whole blood and likely also in cell-free samples such as plasma and cerebrospinal fluid (CSF) secondary to contamination of cellular DNA released from damaged cells.11,12 Distinguishing between HHV-6 infection/reactivation between latent ciHHV-6 is vital in clinical decision making. Reactivation of ciHHV-6 remains a controversial concept and has only been reported on a case report basis.13,14 Its diagnosis requires positive virus culture and genome sequencing to confirm the identity of the isolated viral strain with the integrated virus.2 Additional clinical significance of ciHHV-6 in hematopoietic stem cell transplantation (HSCT) is discussed below.
Given the high prevalence of latent infection and asymptomatic reactivation, difficulties with accurate diagnosis, and data discrepancy in the clinical efficacy of therapeutic and/or prophylaxis antiviral treatment, controversy remains regarding the best approach to treat HHV-6 infection. The most recent guideline on the management of HHV-6 infection in patients with hematological malignancies and after HSCT came from the 2017 European Conference on Infections in Leukemia (ECIL).2 Here, we review the recent advances on the clinical impact and diagnostic techniques of HHV-6 infection in HSCT recipients and discuss the evolving role of antiviral therapy and potential prophylaxis in the post-transplant setting.
Clinical impact of HHV-6 infection in HSCT recipients
Primary HHV-6B infection after HSCT has only been reported in infants, and manifested as fever and rash that may or may not have other end-organ involvement.15,16 In patients older than 2 years of age, new detection of HHV-6 can be assumed to be from virus reactivation universally, either from latent infection (endogenous source, from the recipient) or reinfection (exogenous source, from the donor).2 HHV-6 reactivation is most commonly reported in the first 2–4 weeks after solid organ transplantation or HSCT,17 although late-occurring infection up to 2 years since transplantation has been reported.18 Cord blood transplantation,19,20 HLA-mismatched unrelated donor,21 T cell-depleted allografts,22 certain graft-versus-host disease (GVHD) prophylaxis,6 acute GVHD (aGVHD) and treatment with glucocorticoids23 have been associated with higher risk of developing HHV-6 infection post-HSCT. HHV-6 reactivation/infection has been associated with fever, rash, encephalitis, pneumonitis, hepatitis, and a variety of transplant complications, including delayed/failed engraftment, GVHD, opportunistic infections and ultimately transplant-related death.5,23
HHV-6 encephalitis
HHV-6 encephalitis is the most common cause of encephalitis after alloHSCT and is increasingly recognized as an important transplant complication.2 The diagnosis of HHV-6 encephalitis is made when (1) HHV-6 DNA is detected in CSF or brain tissue coinciding with (2) acute-onset central nervous system (CNS) dysfunction that (3) cannot be explained by other identifiable cause(s).2,24,25 HHV-6 encephalitis is almost exclusively caused by HHV-6B.2,6 Previous literature has reported an incidence of HHV-6 encephalitis ranging widely from 1.0 to 11.6% among alloHSCT recipients.22
The most common presentation of HHV-6 encephalitis is post-transplant acute limbic encephalitis. Symptoms are characterized as delirium, amnesia, confusion, loss of consciousness and seizures.22 Focal neurological deficits are rare.2 CSF analysis is largely unremarkable. Magnetic resonance imaging of the brain may reveal hyperintense lesions involving the bilateral temporal lobes primarily affecting the hippocampus and amygdala on T2-weighted flair sequence22 in approximately 60% of the patients.26 Brain biopsy/autopsy may reveal direct viral invasion into the brain tissue, predominantly in astrocytes and neurons.27 Other characteristic but non-specific findings are necrosis, demyelination and lymphocyte infiltration.25
The prognosis of HHV-6 encephalitis is poor, with a high mortality rate and high likelihood of neurological sequelae among survivors leading to significant functional compromise.22 A review by Zerr on 44 HHV-6 encephalitis cases reported a 28-day disease-specific mortality of 25% and overall mortality of 39%. Additionally, 18% of the patients suffered from lingering neurological compromise.25 In a large-scale national database study by Ogata et al.6 the overall survival on day 100 after HSCT was 58.3% in patients who developed HHV-6 encephalitis and 80% in who did not. Among the survivors who completed antiviral therapy, 57% patients had persistent neuropsychological sequelae. The most common sequelae were memory deficit and temporal lobe epilepsy.6,22
Other end-organ dysfunction associated with HHV-6
A causal association of HHV-6 with other end-organ damage is less well established.2 Recently, HHV-6 has been identified as an emerging cause of interstitial pneumonitis and idiopathic pneumonia in immunocompromised patients.28–30 Among bronchoalveolar lavage (BAL) samples collected from 69 HSCT recipients diagnosed with idiopathic pneumonia syndrome, HHV-6 was the most frequently identified pathogen and was the only pathogen detected in approximately 50% of the pathogen-positive samples. And detection of HHV-6 was associated with increased mortality.28 Another study that included a heterogenous population of patients with hematological malignancies with or without HSCT reported that HHV-6 recovered from BAL fluid is mostly a co-pathogen with other organisms (86%) whose clinical significance remains undetermined at this time. However, in cases where HHV-6 was the only detected pathogen along with evidence of extrapulmonary HHV-6 disease, antiviral therapy may provide substantial clinical benefit.29 Jouneau et al.31 have suggested that HHV-6 polymerase chain reaction (PCR) should be performed on BAL fluid in immunocompromised patients with acute respiratory failure and/or those with unexplained ground glass attenuations on computed tomography scans.
Acute liver failure and acute renal failure associated with HHV-6 have been well documented in liver and kidney transplant patients, respectively.32,33 However, only very limited cases have been reported in post-HSCT patients.34
HHV-6-associated myelosuppression and graft failure
HHV-6 infects hematopoietic cell cells, including progenitor stem cells.35 In vitro study has shown that viral replication can occur throughout the process of hematopoietic differentiation.36 HHV-6 reactivation has been increasingly associated with myelosuppression and graft failure in the post-HSCT setting. In a single-institute prospective study, Imbert-Marcille et al.37 reported that HHV-6 infection was associated with partial or total bone marrow suppression irrespective of the type or source of graft. Hentrich et al.38 reported that 11 of the 96 patients who developed HHV-6 viremia after HSCT suffered from bone marrow suppression, with more than half of them being pancytopenic. Two large retrospective studies reported an association of post-HSCT HHV-6 infection with delayed platelet engraftment and increased requirement of platelet transfusions.5,38 Similar results have also been reported in other cell lineages, including monocytes5 and neutrophils.39 High peripheral blood viral load,39 unmatched donors5 and cord blood transplant40 have been shown to carry a higher risk of HHV-6-associated graft failure.
HHV-6 and delayed T-cell immune reconstitution
Timely CD4+ T-cell immune reconstitution after alloHSCT is associated with improved overall and transplant-related survival.41,42 Previously, studies revealed that delayed or absent CD4+ immune reconstitution increases the risk of reactivation of endogenous latent viruses, including HHV-6.43 On the other hand, HHV-6 primarily targets CD4+ T cells and renders them susceptible to apoptosis.44 The importance of the interplay between HHV-6 reactivation and post-HSCT immune reconstitution has been increasingly recognized over the past few years. Similar to data on adenovirus and Epstein–Barr virus, post-HSCT patients who have adequate CD4+ T-cell immune reconstitution were found to have lower HHV-6 viral load.45 Admiraal et al.43 showed that reactivation of HHV-6 increases the risk of development of aGVHD only in patients without successful CD4+ T-cell immune reconstitution. Very recently, the same group reported that in post-HSCT patients, a high level of HHV-6 viral load is associated with late CD4+ T-cell immune reconstitution (thymus-dependent phase) while early reconstitution is not affected.46 This effect can be reversed by a variety of antiviral therapy, which improved the chance of successful CD4+ immune reconstitution by 42.8%. Notably, this effect also exists in patients who had asymptomatic HHV-6 viremia and received antiviral therapy for other reasons, which raises the question if more aggressive antiviral therapy should be used in post-HSCT HHV-6 viremia, particularly in asymptomatic patients.
HHV-6 and GVHD
Post-HSCT HHV-6 reactivation has been increasingly associated with the development of aGVHD and allograft rejection. In one study where a large-scale PCR assay was used to analyze 13 DNA viruses reactivation in post-HSCT setting, HHV-6 was the most frequently detected virus, and the only one associated with increased risk of aGVHD.47 A large prospective study in alloHSCT recipients found that high HHV-6 viral load (>1000 HHV-6 DNA copies/ml) was associated with subsequent grades II to IV aGVHD, worse overall survival (OS) and higher non-relapse mortality.48 Similar results were observed in a study of 235 patients where patients with HHV-6 viremia had a significantly higher risk of development of aGHVD (47% versus 30%, p = 0.009) and inferior 6-month OS.49 Cord blood transplantation48 and myeloablative conditioning50 have been identified as potential risk factors. Although a direct causal relationship between HHV-6 and aGVHD has not been established, available data suggest that HHV-6 infection is associated with a proinflammatory state which may play an important role in the development of aGVHD.51,52 Studies from HSCT recipients documented an elevated proinflammatory cytokine response (IL-6, IL-2, IFN-γ, et al.), which has also been associated with aGVHD.53 Of note, multiple studies have found that HHV-6 is associated with a particularly high incidence of skin aGVHD.47,48 Since skin rash can also be a symptom of primary HHV-6 acquisition54 or reactivation in the post-HSCT setting,55 caution should be applied to distinguish the rash related to HHV-6 infection from rash related to skin aGVHD. No data are available at this time regarding if antiviral therapy will reduce the incidence of aGVHD in HHV-6 viremia.
HHV-6 and cytomegalovirus reactivation
Similar to findings in solid organ transplant patients, HHV-6 reactivation has been associated with an increased risk of cytomegalovirus (CMV) reactivation post-HSCT.48,56,57 The association of HHV-6 reactivation and CMV disease is less clear. Zerr et al.48 reported that HHV-6 reactivation was independently associated with increased risk of subsequent CMV reactivation. Particularly, high-level HHV-6 reactivation (>1000 copies DNA/ml) was strongly associated with increased risk of high-level CMV reactivation. A study in haploidentical HSCT recipients56 reported that 87% of HHV-6 reactivations were followed by a CMV reactivation, with a median interval of 15 days between the two viruses. The incidence was 14.5-fold higher than HHV-6 non-reactivated patients. Evidence suggests that HHV-6 reactivation might interfere with the function of the host immune system through a variety of mechanisms, including a selective suppression of IL-12 expression, which is a critical cytokine in the generation of Th-1-polarized antiviral immune responses.57,58 Wang et al.59 reported that HHV-6 reactivation was associated with an absence of CMV-specific lymphocyte proliferative response, and patients with persistent HHV-6 viremia were more likely to need repeated courses of preemptive antiviral therapy against CMV within the first 6 months after HSCT.
CiHHV-6 and its clinical impact in the post-HSCT setting
The impact of ciHHV-6 on human disease is largely unknown, due to the rarity of the condition and the diagnostic challenge. Notably, one large registry study reported a three times higher incidence of angina pectoris in individuals with ciHHV-6 which was thought probably related to telomeric disruption or viral gene-stimulated chronic inflammation.60 Data on the post-HSCT population are sparse. A large single-institution retrospective study found aGVHD grades II–IV and CMV viremia were significantly more frequent in HSCT recipients with ciHHV-6 (inherited or from ciHHV-6 donor).3 The detailed mechanisms and clinical significance of these findings remain to be elucidated.
Diagnosis
Diagnosis of HHV-6 infection
Viral culture remains the gold standard of diagnosis by definition.2 However, due to the absence of standardization and sensitivity data, this method is not applicable to routine laboratory HHV6 diagnosis and remains to be used for research purpose only. HHV-6 antigen test by immunohistochemical staining has limited sensitivity and cannot distinguish between HHV-6A and HHV-6B. It is not indicated in HSCT patients.2 Quantitative viral DNA PCR is the mainstay of HHV-6 diagnosis. A variety of real-time PCR assays for HHV-6 DNA load are available, some of which can differentiate between HHV-6A and HHV-6B.61 A WHO International Standard for HHV-6B virus DNA testing is now availabl.62 Assays using reverse transcription PCR (RT-PCR) to detect HHV-6 mRNA are also available.2 Viral DNA testing should be performed in specific clinical samples with regards to various clinical manifestations of the infection. For example, HHV-6B DNA should be tested in CSF or brain tissue if there is clinical suspicion of HHV-6 encephalitis;20 blood and bone marrow should be tested for HHV-6B DNA if there is failed engraftment post-HSCT. It is worth noting that viral DNA in tissue is not necessarily diagnostic and may reflect HHV-6 viremia or local infiltration of HHV-6-infected lymphocytes.2 Thus, when suspecting HHV-6-associated end-organ disease other than encephalitis, myelosuppression, or failed engraftment, one should consider confirmatory test by immunochemistry, in situ hybridization or RT-PCR for mRNA.2
Diagnosis of ciHHV-6
CiHHV-6 can be confirmed by fluorescence in situ hybridization demonstrating HHV-6 integrated into a human chromosome, or by evidence of a ratio of one copy of HHV-6 DNA to cellular genome by droplet digital PCR.63 The hallmark of ciHHV-6 is persistently high levels of HHV-6 viral load in whole blood (>5.5 log10 copies/ml)11 that is refractory to antivirals.64 Routine test for ciHHV-6 in HSCT is not recommended at this time. When necessary, ciHHV-6 can be easily excluded by a negative HHV-6 PCR on a pre-transplant sample from the recipient or a sample from the donor at any time.2 Additionally, for HSCT recipients from ciHHV-6 donor, HHV-6 viral load in blood will increase in parallel with leukocyte engraftment;65 on the other hand, for ciHHV-6 recipients themselves, high viral load will be detected pre-HSCT in blood and will decrease post-HSCT, but will persist in non-hematopoietic tissues.66 Detection of HHV-6 DNA in blood-free samples, that is, hair follicles, nails, et al. happens exclusively in persons with ciHHV-6 and should be performed whenever high viral load persists in the blood without any clinical symptoms or refractory to antivirals (Table 1).67,68 Diagnosis of ciHHV-6 reactivation must be confirmed by positive viral culture and viral genome sequencing to confirm the identity of the viral isolate.2
Table 1.
Distinguishing between HHV-6 reactivation and latent ciHHV-6 post-HSCT.
| HHV-6 reactivation | Donor ciHHV-6 | Recipient ciHHV-6 | |
|---|---|---|---|
| Peak of blood HHV-6 DNA | Variable | With leukocyte engraftment | Pre-HSCT |
| One HHV-6 copy per leukocyte | No | Yes | No |
| One HHV-6 copy per non-hematopoietic cell | No | No | Yes |
| Persistently high blood HHV-6 DNA | Variable | Yes | No |
| Persistently high non-hematopoietic cell HHV-6 DNA | No | No | Yes |
| Response to antiviral therapy | Yes | No | No |
ciHHV-6, chromosomally integrated HHV-6; HSCT, hematopoietic stem cell transplantation.
Treatment
Indication(s)
An increased awareness of clinical conditions associated with HHV-6 reactivation, particularly, in the post-HSCT setting, in recent years has resulted in a growing interest of the best treatment strategy for HHV-6 infection. Treatment of encephalitis associated with HHV-6 is generally indicated and endorsed by American Society of Transplantation Infectious Disease Community of Practice69 and the ECIL.2 Recommendations do not exist for the treatment of HSCT-related clinical entities other than encephalitis that have been associated with HHV-6 reactivation. Many argue against it given the high incidence of “asymptomatic” HHV-6 reactivation, frequent spontaneous resolution of positive HHV-6 PCR, and concern for side effects of antiviral therapies. HHV-6 reactivation used to be felt to be a marker of impaired cellular immunity due to the patients’ underlying hematological malignancies and/or iatrogenic immunodeficiency69 that often coincide other clinical process(es). However, as discussed above, emerging data have provided more insight into the interplay between HHV-6 and transplanted immune system23,45,46 and the adverse impact of HHV-6 on HSCT-related complications and survival.5,38–40,48 A more proactive treatment strategy may be considered. This is particularly supported by data reported by de Koning et al.46 that CD+ T-cell immune reconstitution was significantly improved in patients who received antiviral therapy with or without symptomatic HHV-6 viremia. Randomized trials on antiviral therapy are urgently needed to establish the causality between HHV-6 reactivation and transplant-related endpoints and to determine if prompt reduction of HHV-6 viremia can improve HSCT outcomes. An adequate monitoring and risk stratification strategy needs to be established. It is important to point out that the ciHHV-6 status of the recipient/donor should be considered while making management decisions regarding HHV-6 viremia, especially when patients present with persistently high viral load.70
Treatment options
Most of the HHV-6 treatment experience comes from HHV-6 encephalitis. No specific antiviral therapy has been approved for HHV-6 treatment specifically at this time. A few medications used for CMV infection, namely, nucleoside analog ganciclovir (or its oral version valganciclovir), pyrophosphate analog foscarnet and nucleotide analog cidofovir have all demonstrated in vitro efficacy against HHV-6.71 Variable evaluation methods, viral strains and cell lines have been used in HHV-6 studies, making direct comparison among antiviral compounds somewhat challenging. A review of existing data suggests that foscarnet appears to be the most effective anti-HHV-6 agent in the in vitro setting with an average selectivity index (SI) of 52.4, followed by cidofovir (SI 18.1) and ganciclovir (SI 4.5).72–74 In vitro drug resistance to all three agents has been reported.73–75 Fortunately, in vivo resistance has only been described on a case report basis.76,77 Among the three agents, cidofovir has very limited CNS penetration78 and its clinical usage in HHV-6 encephalitis is only limited to rare case reports.79 Zerr et al.80 first reported a concurrent decrease in serum and CSF HHV-6 viral load with antiviral therapy with foscarnet and/or ganciclovir in post-HSCT patients with HHV-6 encephalopathy. Recently, data comparing foscarnet and ganciclovir from Japan24 showed that the response rates of neurological symptoms were 83.8% and 71.4% with foscarnet and ganciclovir, respectively. The appropriate dosing for HHV-6 treatment has not been well established at this time. Data from the same Japanese study found that both full-dose foscarnet (⩾180 mg/kg) and ganciclovir (⩾10 mg/kg) were associated with better response rate. Additionally, patients who received combination therapy with both foscarnet and ganciclovir at various doses had a response rate of 100%.24 However, most of the data come from small case series, which limits conclusions regarding the superiority of either mono- or combination therapy. Additionally, side effects, particularly the myelosuppressive and nephrotoxic properties of foscarnet and ganciclovir, preclude some post-HSCT patients from receiving these two drugs or dosing titration.2 Novel treatment modalities are urgently needed.
Brincidofovir (CMX-001) is a prodrug of cidofovir that has been proven to be effective against many DNA viruses. It has shown high in vitro efficacy81 and excellent activity in animal models against HHV-6 species with adequate CNS penetration.82 An oral preparation of brincidofovir was found to have profound gastrointestinal toxicity in phase III trials,83 and has since been replaced by an intravenous formulation. Brincidofovir is currently not available for clinical use.
Artesunate is a medication approved for the treatment of severe malaria which has been shown to be effectively against HHV-6 by reducing early and late viral protein synthesis at a relatively safe concentration.84 Hakacova et al.85 reported a case of HHV-6 myocarditis that was successfully treated with artesunate in a pediatric patient.
Cyclopropavir (MBX-400) is a pronucleotide analog that shares similar mechanism of action with ganciclovir is currently being tested in phase II trials for CMV infection. It was found to be extremely effective against both HHV-6A and HHV-6B in vitro71 and may serve as a potential treatment option in the future. Adoptive immunotherapy with HHV-6-specific T cells is a new treatment modality that has been proven to have effective viral killing capacity in vitro86 and in vivo.87
The optimal duration of antiviral therapy is yet to be established. In general, it should be administrated for at least 3 weeks and until clearance of HHV-6 DNA from blood and from CNS, if possible. Dose reduction of immunosuppressive medications should be considered.2
Prophylaxis and monitoring
Routine screening of HHV-6 viremia pre- or post-HSCT is currently not recommended and no consensus has been reached regarding appropriate preventative methods.2 Multiple small-sized prospective non-randomized studies attempted to establish the effective prophylactic or preemptive strategies to prevent HHV-6 reactivation and/or HHV-6 encephalitis with variable results. Ishiyama et al.88 reported that pre-engraftment prophylaxis with foscarnet 90 mg/kg/day might reduce the risk of HHV-6 encephalitis but was not enough to prevent HHV-6 reactivation. Ogata’s team from Japan have trialed foscarnet prophylaxis in HSCT patients at both low (50 mg/kg/day)89 and high (90 mg/kg/day) doses.90 At higher dose,90 foscarnet significantly suppressed systemic HHV-6 reactivation in cord blood transplant recipients but failed to prevent the development of HHV-6 encephalitis or aGVHD. It may have an impact on reducing the severity of HHV-6 encephalitis. Preemptive therapy with ganciclovir91 or foscarnet92 have both been tested and failed to demonstrate a benefit on HHV-6 encephalitis prevention, which was felt to be due the timing of initiation of treatment and the dynamic kinetics of plasma HHV-6 viral load. Recently, subgroup analysis from the SUPPRESS trial, a randomized, double-blind trial of oral brincidofovir for CMV prophylaxis after alloHSCT, demonstrated that brincidofovir may reduce the incidence of HHV-6 viremia in the post-HSCT setting.93 However, it is worth noting that the overall incidence of HHV-6 viremia in this study was low (11% in placebo group) and only one patient developed HHV-6 encephalitis.
With the increased awareness of the interplay between HHV-6 reactivation and the transplant immune system,46,48–50 there has been growing interest in a more intensive monitoring and prophylaxis strategy in the post-HSCT setting. Further clinical investigation is urgently needed to establish the appropriate timing and dosing for specific antiviral agents.
BK virus
Overview
One of the most insidious viral infections that affect immunocompromised patients post-HSCT is BK virus. BK virus is a member of the polyomavirus family, along with JC virus. Polyomaviruses are double-stranded DNA viruses that often result in infection in patients with dampened immune systems. BK virus was first identified in 1971 in a patient who underwent renal transplant.94 It was identified as a virus that could cause graft loss in renal transplant patients.95 BK virus can remain latent in the kidney and can become reactivated in the setting of immunosuppression after transplantation of stem cells or solid organs. BK-associated nephropathy results in significant morbidity and mortality.
The BK virus has multiple subtypes including genotype I though VI. The geographic distribution pattern differs among the different subgroups of BK virus.94 Four different BK virus genotypes have been described.95 The two major proteins in the BK virus genome are the large T antigen and the small t antigen.95 It has been estimated that 80% of immunocompetent persons have positive BK serology. The acquisition of BK virus can be through the genitourinary tract (via sexual transmission) or via respiratory transmission. The major route of transmission is respiratory.96
The ECIL has developed formalized guidelines for the prevention, diagnosis, and treatment of BK viral infection in the setting of allogeneic transplant.97 These recommendations stem from a joint venture of multiple expert groups who evaluate evidence-based information. In summary, there is minimal role for interventions in prevention of BK virus, but there are some data to suggest a role for antiviral medications or cell-based therapies in the treatment of BK virus infection. These aspects are discussed in detail herein.
Clinical impact of BK virus on HSCT recipients
Hemorrhagic cystitis due to BK virus most often occurs within the first 3 months post-HSCT. Symptoms include, but are not limited to, hematuria, frequency, and urgency. Hemorrhagic cystitis has been reported at a frequency of 5–70%.98 Often, patients have BK viremia or viruria, but are asymptomatic. The incidence of hemorrhagic cystitis has been reported to be 24% over a 2-year period in the post-transplant setting. The median time of onset is 45 days.99 The time to clearance was reported to be 21 days. The time course of hemorrhagic cystitis can sometimes suggest the etiology: cyclophosphamide causes hemorrhagic cystitis generally within 7 days compared with BK virus which causes late-onset hemorrhagic cystitis. It has been reported that BK virus can lead to interstitial nephritis if it progresses, but it is also important to rule out other causes of interstitial nephritis in patients who have undergone transplantation.100
There seems to be a small risk for urothelial cancer with BK infection, though the data are not very convincing.101 Polyomavirus family members have been found in 4% of urothelial tract cancers. The risk for invasive bladder cancer is 4–5 times higher in patients with BK virus infection.102 The reason for the higher risk of malignancy may be related to inflammation induced by BK virus in the genitourinary tract, but this has not been definitively shown.
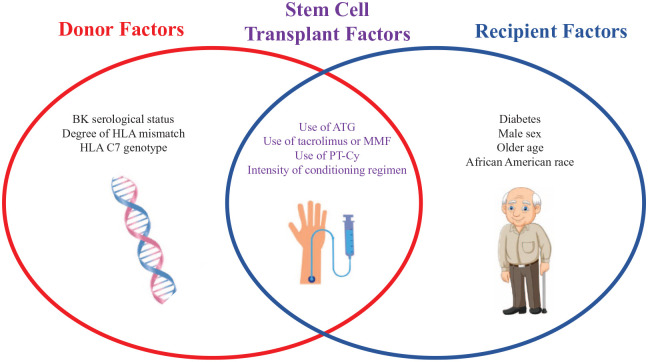
Risk factors for BK virus infection in the setting of stem cell transplantation include the type of immunosuppression used, the intensity of the conditioning regimen, the choice of donor with regards to donor BK serological status, male gender, and the CMV status.94 The greater degree of immunosuppression correlates with the risk for developing BK virus infection. Prolonged and profound immunosuppression can contribute to leukopenia including neutropenia and lymphopenia, which predispose patients to viral infection. Prolonged cold ischemia (for renal transplant recipients) and ureteral stent placement have been proposed as risk factors but are not very well established (Figure 1).
Figure 1.
Various risk factor for BK virus infection.
Diagnosis
The diagnosis of BK virus infection is often made via assessment of urine and plasma for BK virus DNA. The diagnosis of BK cystitis, per ECIL guidelines, is made when a patient has gross hematuria and a urine BK viral load in excess of 70 million copies/ml.97 Other etiologies for hematuria must be excluded. The dynamics and frequency of BK virus DNA in urine and in blood after stem cell transplant are important considerations. In patients who have received stem cell transplant, the sensitivity and negative predictive value of BK virus in the urine are high at a threshold of greater than 10 million copies/ml.97 It has been proposed that BK viruria has a higher specificity and positive predictive value at a threshold of 1000–10,000 copies/ml. In a pediatric study, the sensitivity of urine BK virus above 10 million copies/ml was 86% and specificity was 60%. This is compared with a sensitivity of 100% and specificity of 86% for blood BK viral load above 1000 copies/ml.103
The pathologic diagnosis can be confounded by concerns for acute cellular rejection, which is why this is mostly important for renal transplant recipients as it often presents similarly (histologically) to BK viral cytopathic changes.104 However, the risk for renal biopsy often outweighs the benefits in patients who are status post-allogeneic stem cell transplant, given leukopenia and thrombocytopenia which may place patients at risk for infection and bleeding. Noninvasive tests are therefore preferred in the practical setting when information is needed in a timely manner.
Screening and prevention
Screening guidelines are not as well established for allogeneic stem cell transplant recipients as compared with renal transplant recipients. KDIGO 2009 guidelines provide important screening information for renal transplant recipients, but further studies are warranted for treatment of BK viral infection in recipients of allogeneic stem cell transplant.105 Prevention of BK viral infection is essential, and intravenous hydration and bladder irrigation have been proposed, though the ECIL does not strongly recommend these interventions. There was no significant improvement in overall risk for hemorrhagic cystitis risk with either hyperhydration or bladder irrigation based on multiple studies.97
Therapy
Decisions about management and treatment of BK virus infection are subject to much interpretation, but the management generally involves restoration of the adaptive immune response. CD4(+) and CD8(+) T cells play a fundamental role in eliminate of BK viremia and viruria. Allogeneic stem cell transplant recipients often have a severely compromised immune system during a defined period of time after conditioning chemotherapy, and the risk of BK infection is very high during this time. Many cases of BK hemorrhagic cystitis are self-limited and symptoms resolve on their own, and not all cases require treatment. For cases that do require intervention, the first and most important measure is tapering immunosuppressive medications if possible. Common strategies include gradual tapering of tacrolimus and/or mycophenylate mofetil (MMF) as long as GVHD does not pose significant problems. Dose reductions of tacrolimus to achieve a goal of 3–4 ng/ml are commonly employed.
A variety of antiviral medications for BK virus have been studied in clinical trials, but the data have not shown clear benefit to the start of antiviral therapy. A systematic review of 40 studies of the use of antiviral medications has not shown an improvement in overall graft survival with the use of antiviral medications.106 Antiviral medications such as cidofovir have been used, though renal toxicity and myelosuppression often limits their use. Fluoroquinolones like ciprofloxacin have been used with some effect.107 The use of intravenous immunoglobulin is a supportive strategy that can help provide humoral immunity.108 Leflunomide has been studied but the current data are not very convincing.109 In stem cell transplant patients, we favor the use of cidofovir (despite the lack of robust data) given that BK viremia can pose significant challenges and complications in this vulnerable patient population. However, creatinine can increase after cidofovir treatment: a retrospective study has shown a trend toward acute kidney injury with increase in serum creatinine by 27%, and renal failure occurred in 40% of stem cell transplant recipients receiving cidofovir.110 Renal function must be carefully monitored while on cidofovir.
In renal transplant patients, management of BK viral infection often begins with screening (secondary prevention). Monthly screenings are performed in the first 6 months post-transplant, per KDIGO 2009 guidelines.105 After this, screening is performed every 3 months until about 2 years post-transplant. For allogeneic stem cell transplant recipients, screening guidelines are not as well established. Further studies are warranted for treatment of BK viral infection in recipients of allogeneic stem cell transplant.
Cytotoxic T lymphocyte (CTL) therapy for treatment of BK virus has been explored, but the data are limited. It has been found that BK virus-specific CTLs are present at low frequencies in the circulation of patients. These cells can express antiviral cytokines including IL-2 and IFN-gamma.111 However, the cytotoxic function of these cells is limited as they do not readily express granzymes. Efforts have been made to rapidly generate CTLs with specificity for BK virus in the clinical setting. Monocyte-derived dendritic cells can be stimulated with viral-specific peptides, which induce the production of virus-specific CTLs. These CD4(+)CD8(+) CTLs can respond readily to BK virus peptides and produce multiple cytokines that help eliminate antigen-coated targets.112 Polyclonal CD4(+)CD8(+) CTLs have been successfully generated against BK antigens, and adoptive transfer of these virus-specific CTLs has been proposed to readily combat BK virus in the clinical setting.113 The virus-specific CTLs (generated by BK viral peptide mixtures) may maintain a memory T response which allows for response to recurring BK viral infection.114 However, there is no clear consensus about the clinical use of virus-specific T-cell products as of 2021.
Adenovirus
Introduction
Adenovirus is a group of DNA viruses that leads to infections most commonly in children between the ages of 6 months to 5 years.115 It cases the epidemic keratoconjunctivitis, pharyngitis, upper respiratory tract infections and gastroenteritis in healthy children and adults.116 The infection is usually self-limited and resolved within 14 days.117 Adenovirus is reported to cause about 5–10% of all febrile illnesses in healthy infants and young children.118 There are over 50 serotypes that can lead to an infection in humans. Its infections are usually self-limited and or asymptomatic. Serotype 5 is most associated with symptomatic disease, including fatal hepatic necrosis and pneumoniae. Fatal adenovirus infections occur most commonly in immunocompromised hosts.119–121
Clinical presentation
The clinical presentation of adenovirus varies widely, depending on age of the patient and the immunocompetence of the host. Adenovirus infections are usually self-limited and or asymptomatic in the immunocompetent host. In an immunocompetent host it causes keratoconjunctivitis, phyaryngitis, upper respiratory tract infection and gastroenteritis. Adenovirus has been rarely the leading infectious organism causing meningitis or encephalitis,122 or myocarditis.123
Disseminated adenovirus infection has been reported in both immunocompetent and immunocompromised hosts, with higher incidence in the latter.124,125
Adenovirus diseases are well identified and characterized in hematopoietic cell transplant (HCT) recipients.120,126,127 Adenoviruses exhibit a wide spectrum of infections in such hosts, and can range from asymptomatic disease to fatal disseminated disease. They can lead to a wide array of infections, including but not limited to: pneumoniae, colitis, hemorrhagic cystitis and tubulointerstitial nephritis.125,127–129
Less common manifestations include hepatitis,129 myocarditis,123 encephalitis124 and disseminated disease with multiorgan failure. Disseminated disease is rare, occurring in 1–7% of cases, with reported mortality rates of 8–26%.130
In addition, the infection can occur due to different modalities, whether a primary infection, reactivation of latent infection in the transplant recipient, or reactivation of infection transmitted in the donated organ.131 Reactivation of adenovirus infection is common following HCT but rarely causes severe disease.
Risk factors
Risk factors for adenovirus include HCT patients treated with T-cell-depleted bone marrow grafts and more intensive immunosuppressive regimens.126,132,133 Others include severe GVHD requiring high dose of steroids,134,135 alemtuzumab, anti-thymocyte globulin and allogenic transplant.136 Allogenic recipients and recipients of unrelated or mismatched grafts have an increased risk of infection.130,137 Interestingly, a review of adenovirus risk factors showed that younger age was the most significant predisposing factor for adenovirus infection, and aGVHD ⩾ grade II for adenovirus disease.133,134,138,139
Diagnosis
Various methods are available to diagnose adenovirus infection. Viral culture, adenovirus-specific viral antigen assays and PCR assays are used most frequently. Adenoviruses are relatively stable and can be readily recovered from various tissue and body fluids samples early during the disease.
Direct detection of adenovirus antigens can be performed with immunologic technology like adenovirus-specific enzyme immunoassay or immunofluorescence assay. A routine test used for other viruses, PCR testing can also be used to detect adenovirus infection. It has a high sensitivity and specificity, though it is difficult to interpret as it may represent virus shedding rather than acute infection.140 Adenovirus viral load can help monitor response to treatment in cases of viremia and assess the risk of an invasive disease.141,142 Screening for adenovirus infection with stool PCR has been well established prior to transplant and in the post-transplant period to improve outcomes in the pediatric allo-transplant settings.143 Detection of adenovirus above a certain threshold in the post-transplant period was shown to precede the onset of viremia.144,145 While screening in the pediatric population is well established, data for adult allo-transplant are still quite diverse and need further studies.130,133,144,146,147
Adenovirus viral load can help monitor response to treatment in cases of viremia and assess the risk of invasive disease.141,142 Because adenoviruses may be shed asymptomatically in throat, stool or urine, it is often necessary to obtain tissue to diagnose some types of disease. Definitive diagnosis of adenovirus disease may require tissue biopsy.
Graft-versus-host disease
Adenovirus pathogenicity and its relationship with the development of GVHD have not been established. Certain authors suggest that with concurrent aGVHD, adenovirus activation carries a significantly poorer prognosis and an increased risk of disseminated disease.148 A single case report found that fluctuations of adenovirus load in leukocytes and urine have an inverse association with concurrent GVHD.149 Moreover, a certain cut-off level of adenovirus viral load, in serum or stool, was found in patients presenting with GVHD compared with patients who did not develop GVHD.150
A review paper showed that the majority of patients with adenovirus infection developed or suffered from aGVHD (grades II–IV). Some 75% of GVHD episodes have been refractory to steroids, requiring a second-line immunosuppressive treatment. The same paper showed that the rate of aGVHD II–IV in patients with asymptomatic infection (33%) was significantly lower than in patients with localized (89%) or disseminated disease (84%).138 On the other hand, it has been suggested that in infections caused by adenovirus, hemorrhagic cystitis was not associated with GVHD.151
T-cell immunity is critical for recovery from adenovirus infection following HSCT. Although early diagnosis and treatment of adenovirus infections in this patient population may improve outcomes, lymphocyte reconstitution also appears crucial for recovery from disease.152,153 Pilot studies of adoptive transfer of T-cell immunity have been performed in children with adenovirus infection after stem cell transplantation.154,155
Prophylaxis
In terms of prophylaxis, vaccination and infection control measures have been implied in the prevention of adenovirus infection.156,157 Available adenovirus vaccines are live attenuated, and such vaccines are preferably avoided in patients with ongoing immunosuppression, such as transplant patients.158 Prevention of adenovirus infection or GVHD in immunocompromised hosts has been limited to meta-analysis suggesting gastrointestinal adenovirus PCR testing prior to transplant, as it predicted the development of intestinal GVHD. Not only aGVHD, but also viral reactivation infections can precede chronic GVHD and are significantly linked to an impaired immune recovery.159
Treatment
In 1996 cidofovir changed the landscape for treatment of CMV infection.160 Since then, its use has expanded for adenovirus infections as well.161–163 Cidofovir nephrotoxicity has always been a major limiting toxicity.164,165 In hematopoietic stem cell and lung transplant recipients, cidofovir therapy has been associated with clinical improvement and a suggestion of increased survival.165,166
The high prevalence of adenovirus infection in HSCT recipients pre- and post-transplantation was significantly related to GVHD symptoms, highlighting the important pathogenic role of these viral infections in clinical complications post-HSCT.167
Brincidofovir is a relatively new investigational agent that has been studied for treatment of adenovirus. It is an orally bioavailable lipid conjugate of cidofovir that has in vitro activity against adenoviruses and other DNA viruses.168,169 Interestingly, brincidofovir is less nephrotoxic compared with the original parent medication cidofovir.170 One concerning adverse event that was reported with in a brincidofovir trial by Marty et al.83 was diarrhea. Diarrhea has been reported as the most common adverse event, with an incidence of 60% compared with placebo arm. Post hoc safety analysis indicated the risk of having diarrhea grade 2 or more with brincidofovir was 73% compared with 34% with placebo.
As T-cell immunity is critical for recovery from adenovirus infection, studies are ongoing for the use of engineered T cells directed against adenovirus.171 An NIH trial evaluating adenovirus-specific CTLs for refractory adenovirus infection is ongoing.
Conclusions
HHV-6, BK virus and adenovirus can be detected in many patients after an allogeneic transplant. This is due to many factors. The age at transplant has been increasing, options for alternative stem cell sources (cord blood as well as haploidentical transplant) have been expanding, transplant recipients have deeper immunosuppression at the time of transplant owing to multiple lines of salvage, changes to conditioning regimens and overall increased availability of testing by PCR for these viremias are among the factors responsible for this (see also Figure 1). The viruses described here lead to a variable clinical course ranging from harmless viremia (cleared by engrafting cells) to more serious infections with end-organ damage (i.e. HHV-6 encephalitis, severe hemorrhagic cystitis, graft failure etc.). Furthermore some of these viruses seem to “leave” a deeper immune defect, which can lead to downstream events inclusive of higher risk of GVHD. More intense infections lead to endothelial injury and subsequent complement activation, resulting in some unique transplant-related syndromes such as thrombotic microangiopathy (TMA) and refractory GVHD (with TMA component), and in these instances antiviral therapy and/or withdrawal off immunosuppression are no longer effective. Morbidity and mortality of such syndromes is rather high, and this further underscores the need for better understanding of the earlier stages of the viremias as they often lead to further post-transplant complications and inferior transplant outcomes. Physicians need to be aware of the potential associations. Further study is urgently needed to better elucidate the pathophysiology of virus-associated clinical conditions. High-quality, randomized studies are warranted to demonstrate if more aggressive monitoring and treatment strategy will reduce the incidence of end-organ infections and other HSCT-related complications and ultimately improve HSCT outcomes.
Footnotes
Author contributions: XW: collected data, wrote manuscript, reviewed and approved final version of the manuscript; SP: collected data, wrote manuscript, reviewed and approved final version of the manuscript; MH: collected data, wrote manuscript, reviewed and approved final version of the manuscript; JC: concept of the manuscript, collected data, wrote manuscript, reviewed and approved final version of the manuscript. There is no overlap with our other published works.
Conflict of interest statement: Dr Jan Cerny serves in the advisory board/consultancy for Jazz Pharmaceuticals and Amgen. He is a Data and Safety Monitoring Board Member for AlloVir, and holds stocks from Actinium Pharmaceuticals, Bluebird Bio Inc., Dynavax Pharma, Atyr Pharmac, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart Inc, and Veru Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our study did not require an ethical board approval because it is a review article.
ORCID iD: Jan Cerny  https://orcid.org/0000-0002-6602-5505
https://orcid.org/0000-0002-6602-5505
Contributor Information
Xin Wang, Department of Medicine, UMass Memorial Medical Center, Worcester, MA, USA.
Shyam A. Patel, Division of Hematology-Oncology, Department of Medicine, UMass Memorial Medical Center, Worcester, MA, USA
Michael Haddadin, Division of Hematology-Oncology, Department of Medicine, UMass Memorial Medical Center, Worcester, MA, USA.
Jan Cerny, Division of Hematology and Oncology, Department of Medicine, UMass Memorial Medical Center, 55 Lake Avenue North, Worcester, MA, 01655, USA.
References
- 1. Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation 2008; 86: 1327–1339. [DOI] [PubMed] [Google Scholar]
- 2. Ward KN, Hill JA, Hubacek P, et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica 2019; 104: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hill JA, Magaret AS, Hall-Sedlak R, et al. Outcomes of hematopoietic cell transplantation using donors or recipients with inherited chromosomally integrated HHV-6. Blood 2017; 130: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol 2014; 159: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zerr DM, Corey L, Kim HW, et al. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis 2005; 40: 932–940. [DOI] [PubMed] [Google Scholar]
- 6. Ogata M, Satou T, Kadota J, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis 2013; 57: 671–681. [DOI] [PubMed] [Google Scholar]
- 7. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev 2005; 18: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Bourgeois A, Labopin M, Guillaume T, et al. Human herpesvirus 6 reactivation before engraftment is strongly predictive of graft failure after double umbilical cord blood allogeneic stem cell transplantation in adults. Exp Hematol 2014; 42: 945–954. [DOI] [PubMed] [Google Scholar]
- 9. Daibata M, Taguchi T, Nemoto Y, et al. Inheritance of chromosomally integrated human herpesvirus 6 DNA. Blood 1999; 94: 1545–1549. [PubMed] [Google Scholar]
- 10. Pellett PE, Ablashi DV, Ambros PF, et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 2012; 22: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward KN, Leong HN, Nacheva EP, et al. Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol 2006; 44: 1571–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward KN, Hoe NL, Thiruchelvam AD, et al. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol 2007; 45: 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Politikos I, McMasters M, Bryke C, et al. Possible reactivation of chromosomally integrated human herpesvirus 6 after treatment with histone deacetylase inhibitor. Blood Adv 2018; 2: 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnafous P, Marlet J, Bouvet D, et al. Fatal outcome after reactivation of inherited chromosomally integrated HHV-6A (iciHHV-6A) transmitted through liver transplantation. Am J Transplant 2018; 18: 1548–1551. [DOI] [PubMed] [Google Scholar]
- 15. Lau YL, Peiris M, Chan GCF, et al. Primary human herpes virus 6 infection transmitted from donor to recipient through bone marrow infusion. Bone Marrow Transplant 1998; 21: 1063–1066. [DOI] [PubMed] [Google Scholar]
- 16. Muramatsu H, Watanabe N, Matsumoto K, et al. Primary infection of human herpesvirus-6 in an infant who received cord blood SCT. Bone Marrow Transplant 2009; 43: 83–84. [DOI] [PubMed] [Google Scholar]
- 17. Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis 2006; 193: 68–79. [DOI] [PubMed] [Google Scholar]
- 18. Rosenfeld CS, Rybka WB, Weinbaum D, et al. Late graft failure due to dual bone marrow infection with variants A and B of human herpesvirus-6. Exp Hematol 1995; 23: 626–629. [PubMed] [Google Scholar]
- 19. Hill JA, Koo S, Guzman Suarez BB, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant 2012; 18: 1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheurer ME, Pritchett JC, Amirian ES, et al. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant 2013; 48: 574–580. [DOI] [PubMed] [Google Scholar]
- 21. Muta T, Fukuda T, Harada M. Human herpesvirus-6 encephalitis in hematopoietic SCT recipients in Japan: a retrospective multicenter study. Bone Marrow Transplant 2009; 43: 583–585. [DOI] [PubMed] [Google Scholar]
- 22. Ogata M, Fukuda T, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant 2015; 50: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 23. Greco R, Crucitti L, Noviello M, et al. Human herpesvirus 6 infection following haploidentical transplantation: immune recovery and outcome. Biol Blood Marrow Transplant 2016; 22: 2250–2255. [DOI] [PubMed] [Google Scholar]
- 24. Ogata M, Oshima K, Ikebe T, et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2017; 52: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 25. Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol 2006; 37(Suppl. 1): S52–S56. [DOI] [PubMed] [Google Scholar]
- 26. Noguchi T, Yoshiura T, Hiwatashi A, et al. CT and MRI findings of human herpesvirus 6–associated encephalopathy: comparison with findings of herpes simplex virus encephalitis. Am J Roentgenol 2010; 194: 754–760. [DOI] [PubMed] [Google Scholar]
- 27. Drobyski WR, Knox KK, Majewski D, et al. Fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med 1994; 330: 1356–1360. [DOI] [PubMed] [Google Scholar]
- 28. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125: 3789–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mariotte E, Schnell D, Scieux C, et al. Significance of herpesvirus 6 in BAL fluid of hematology patients with acute respiratory failure. Infection 2011; 39: 225–230. [DOI] [PubMed] [Google Scholar]
- 30. Ishio T, Endo T, Okada K, et al. Human herpesvirus-6 pneumonitis around the engraftment of cord blood transplantation following foscarnet prophylaxis in a patient with acute leukemia. Case Rep Hematol 2015; 2015: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jouneau S, Poineuf JS, Minjolle S, et al. Which patients should be tested for viruses on bronchoalveolar lavage fluid? Eur J Clin Microbiol Infect Dis 2013; 32: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buyse S, Roque-Afonso A-M, Vaghefi P, et al. Acute hepatitis with periportal confluent necrosis associated with human herpesvirus 6 infection in liver transplant patients. Am J Clin Pathol 2013; 140: 403–409. [DOI] [PubMed] [Google Scholar]
- 33. Tong CYW, Muir P, Bakran A, et al. Re: the association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation 2003; 76: 621–622. [DOI] [PubMed] [Google Scholar]
- 34. Hill JA, Myerson D, Sedlak RH, et al. Hepatitis due to human herpesvirus 6B after hematopoietic cell transplantation and a review of the literature. Transpl Infect Dis 2014; 16: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luppi M, Barozzi P, Morris C, et al. Human herpesvirus 6 latently infects early bone marrow progenitors in vivo. J Virol 1999; 73: 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andre-Garnier E, Milpied N, Boutolleau D, et al. Reactivation of human herpesvirus 6 during ex vivo expansion of circulating CD34+ haematopoietic stem cells. J Gen Virol 2004; 85: 3333–3336. [DOI] [PubMed] [Google Scholar]
- 37. Imbert-Marcille BM, Tang XW, Lepelletier D, et al. Human herpesvirus 6 infection after autologous or allogeneic stem cell transplantation: a single-center prospective longitudinal study of 92 patients. Clin Infect Dis 2000; 31: 881–886. [DOI] [PubMed] [Google Scholar]
- 38. Hentrich M, Oruzio D, Jäger G, et al. Impact of human herpesvirus-6 after haematopoietic stem cell transplantation. Br J Haematol 2005; 128: 66–72. [DOI] [PubMed] [Google Scholar]
- 39. Ljungman P, Wang F-Z, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol 2000; 111: 774–781. [PubMed] [Google Scholar]
- 40. Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant 2010; 45: 1204–1211. [DOI] [PubMed] [Google Scholar]
- 41. Fedele R, Martino M, Garreffa C, et al. The impact of early CD4+ lymphocyte recovery on the outcome of patients who undergo allogeneic bone marrow or peripheral blood stem cell transplantation. Blood Transfus 2012; 10: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Admiraal R, Nierkens S, de Witte MA, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol 2017; 4: e183–e191. [DOI] [PubMed] [Google Scholar]
- 43. Admiraal R, de Koning CCH, Lindemans CA, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol 2017; 140: 1643–1650.e9. [DOI] [PubMed] [Google Scholar]
- 44. Yasukawa M, Inoue Y, Ohminami H, et al. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection. J Gen Virol 1998; 79: 143–147. [DOI] [PubMed] [Google Scholar]
- 45. Dagna L, Pritchett JC, Lusso P. Immunomodulation and immunosuppression by human herpesvirus 6A and 6B. Future Virol 2013; 8: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Koning C, Admiraal R, Nierkens S, et al. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv 2018; 2: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inazawa N, Hori T, Hatakeyama N, et al. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol 2015; 87: 1427–1435. [DOI] [PubMed] [Google Scholar]
- 48. Zerr DM, Boeckh M, Delaney C, et al. HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1700–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dulery R, Salleron J, Dewilde A, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol Blood Marrow Transplant 2012; 18: 1080–1089. [DOI] [PubMed] [Google Scholar]
- 50. Aoki J, Numata A, Yamamoto E, et al. Impact of human herpesvirus-6 reactivation on outcomes of allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015; 21: 2017–2022. [DOI] [PubMed] [Google Scholar]
- 51. Ogata M, Satou T, Kawano R, et al. Correlations of HHV-6 viral load and plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem cell transplant recipients. Bone Marrow Transplant 2010; 45: 129–136. [DOI] [PubMed] [Google Scholar]
- 52. Fujita A, Ihira M, Suzuki R, et al. Elevated serum cytokine levels are associated with human herpesvirus 6 reactivation in hematopoietic stem cell transplantation recipients. J Infect 2008; 57: 241–248. [DOI] [PubMed] [Google Scholar]
- 53. Mohty M, Blaise D, Faucher C, et al. Inflammatory cytokines and acute graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Blood 2005; 106: 4407–4411. [DOI] [PubMed] [Google Scholar]
- 54. Yoshikawa T, Ihira M, Ohashi M, et al. Correlation between HHV-6 infection and skin rash after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 28: 77–81. [DOI] [PubMed] [Google Scholar]
- 55. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005; 352: 768–776. [DOI] [PubMed] [Google Scholar]
- 56. Crocchiolo R, Giordano L, Rimondo A, et al. Human herpesvirus 6 replication predicts cytomegalovirus reactivation after allogeneic stem cell transplantation from haploidentical donor. J Clin Virol 2016; 84: 24–26. [DOI] [PubMed] [Google Scholar]
- 57. Lusso P. HHV-6 and the immune system: mechanisms of immunomodulation and viral escape. J Clin Virol 2006; 37(Suppl. 1): S4–S10. [DOI] [PubMed] [Google Scholar]
- 58. Smith A, Santoro F, Di Lullo G, et al. Selective suppression of IL-12 production by human herpesvirus 6. Blood 2003; 102: 2877–2884. [DOI] [PubMed] [Google Scholar]
- 59. Wang F-Z, Larsson K, Linde A, et al. Human herpesvirus 6 infection and cytomegalovirus-specific lymphoproliferative responses in allogeneic stem cell transplant recipients. Bone Marrow Transplant 2002; 30: 521–526. [DOI] [PubMed] [Google Scholar]
- 60. Gravel A, Dubuc I, Morissette G, et al. Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc Natl Acad Sci U S A 2015; 112: 8058–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cassina G, Russo D, De Battista D, et al. Calibrated real-time polymerase chain reaction for specific quantitation of HHV-6A and HHV-6B in clinical samples. J Virol Methods 2013; 189: 172–179. [DOI] [PubMed] [Google Scholar]
- 62. NIBSC. WHO International Standard 1st WHO International Standard for HHV-6B virus DNA NIBSC code: 15/266 instructions for use (Version 3.0, Dated 23/11/2017), https://www.nibsc.org/documents/ifu/15-266.pdf (accessed 2 January 2021).
- 63. Sedlak RH, Cook L, Huang M-L, et al. Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Clin Chem 2014; 60: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hubacek P, Maalouf J, Zajickova M, et al. Failure of multiple antivirals to affect high HHV-6 DNAaemia resulting from viral chromosomal integration in case of severe aplastic anaemia. Haematologica 2007; 92: e98–e100. [DOI] [PubMed] [Google Scholar]
- 65. Purev E, Winkler T, Danner RL, et al. Engraftment of donor cells with germ-line integration of HHV6 mimics HHV6 reactivation following cord blood/haplo transplantation. Blood 2014; 124: 1198–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jeulin H, Guéry M, Clément L, et al. Chromosomally integrated HHV-6: slow decrease of HHV-6 viral load after hematopoietic stem-cell transplantation. Transplantation 2009; 88: 1142–1143. [DOI] [PubMed] [Google Scholar]
- 67. Tanaka-Taya K, Sashihara J, Kurahashi H, et al. Human herpesvirus 6 (HHV-6) is transmitted from parent to child in an integrated form and characterization of cases with chromosomally integrated HHV-6 DNA. J Med Virol 2004; 73: 465–473. [DOI] [PubMed] [Google Scholar]
- 68. Hubacek P, Muzikova K, Hrdlickova A, et al. Prevalence of HHV-6 integrated chromosomally among children treated for acute lymphoblastic or myeloid leukemia in the Czech Republic. J Med Virol 2009; 81: 258–263. [DOI] [PubMed] [Google Scholar]
- 69. Pellett Madan R, Hand J. Human herpesvirus 6, 7, and 8 in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. Epub ahead of print 4 April 2019. DOI: 10.1111/ctr.13518. [DOI] [PubMed] [Google Scholar]
- 70. Epstein DJ, Tan SK, Deresinski S. HHV-6 and septic shock: tenuous proof of causation. Am J Transplant 2019; 19: 303. [DOI] [PubMed] [Google Scholar]
- 71. Prichard MN, Whitley RJ. The development of new therapies for human herpesvirus 6. Curr Opin Virol 2014; 9: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. HHV-6 Foundation. HHV-6 Antivirals. HHV-6 disease information for patients, clinicians, and researchers. Apply for a Grant, https://hhv-6foundation.org/research/hhv-6-antiviral-drug-resistance (accessed 2 January 2021).
- 73. Manichanh C, Olivier-Aubron C, Lagarde JP, et al. Selection of the same mutation in the U69 protein kinase gene of human herpesvirus-6 after prolonged exposure to ganciclovir in vitro and in vivo. J Gen Virol 2001; 82: 2767–2776. [DOI] [PubMed] [Google Scholar]
- 74. Bonnafous P, Naesens L, Petrella S, et al. Different mutations in the HHV-6 DNA polymerase gene accounting for resistance to foscarnet. Antivir Ther 2007; 12: 877–888. [PubMed] [Google Scholar]
- 75. Bonnafous P, Boutolleau D, Naesens L, et al. Characterization of a cidofovir-resistant HHV-6 mutant obtained by in vitro selection. Antiviral Res 2008; 77: 237–240. [DOI] [PubMed] [Google Scholar]
- 76. Isegawa Y, Hara J, Amo K, et al. Human herpesvirus 6 ganciclovir-resistant strain with amino acid substitutions associated with the death of an allogeneic stem cell transplant recipient. J Clin Virol 2009; 44: 15–19. [DOI] [PubMed] [Google Scholar]
- 77. Baldwin K. Ganciclovir-resistant human herpesvirus-6 encephalitis in a liver transplant patient: a case report. J Neurovirol 2011; 17: 193–195. [DOI] [PubMed] [Google Scholar]
- 78. Upadhyayula S, Michaels MG. Ganciclovir, foscarnet, and cidofovir: antiviral drugs not just for cytomegalovirus. J Pediatric Infect Dis Soc 2013; 2: 286–290. [DOI] [PubMed] [Google Scholar]
- 79. Denes E, Magy L, Pradeau K, et al. Successful treatment of human herpesvirus 6 encephalomyelitis in immunocompetent patient. Emerg Infect Dis 2004; 10: 729–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zerr DM, Gupta D, Huang ML, et al. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34: 309–317. [DOI] [PubMed] [Google Scholar]
- 81. Williams-Aziz SL, Hartline CB, Harden EA, et al. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother 2005; 49: 3724–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bidanset DJ, Beadle JR, Wan WB, et al. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J Infect Dis 2004; 190: 499–503. [DOI] [PubMed] [Google Scholar]
- 83. Marty FM, Winston DJ, Chemaly RF, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019; 25: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Milbradt J, Auerochs S, Korn K, et al. Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J Clin Virol 2009; 46: 24–28. [DOI] [PubMed] [Google Scholar]
- 85. Hakacova N, Klingel K, Kandolf R, et al. First therapeutic use of artesunate in treatment of human herpesvirus 6B myocarditis in a child. J Clin Virol 2013; 57: 157–160. [DOI] [PubMed] [Google Scholar]
- 86. Gerdemann U, Keukens L, Keirnan JM, et al. Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood 2013; 121: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014; 6: 242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ishiyama K, Katagiri T, Ohata K, et al. Safety of pre-engraftment prophylactic foscarnet administration after allogeneic stem cell transplantation. Transpl Infect Dis 2012; 14: 33–39. [DOI] [PubMed] [Google Scholar]
- 89. Ogata M, Satou T, Inoue Y, et al. Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transplant 2013; 48: 257–264. [DOI] [PubMed] [Google Scholar]
- 90. Ogata M, Takano K, Moriuchi Y, et al. Effects of prophylactic foscarnet on human herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: a prospective multicenter trial with an historical control group. Biol Blood Marrow Transplant 2018; 24: 1264–1273. [DOI] [PubMed] [Google Scholar]
- 91. Ogata M, Satou T, Kawano R, et al. Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant 2008; 41: 279–285. [DOI] [PubMed] [Google Scholar]
- 92. Ishiyama K, Katagiri T, Hoshino T, et al. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant 2011; 46: 863–869. [DOI] [PubMed] [Google Scholar]
- 93. Hill JA, Zerr D, Nichols G, et al. Oral brincidofovir decreased HHV-6 viremia in hematopoietic cell transplant recipients: results from the suppress study. Biol Blood Marrow Transplant 2019; 25: S358. [Google Scholar]
- 94. Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant 2015; 30: 209–217. [DOI] [PubMed] [Google Scholar]
- 95. Chong S, Antoni M, Macdonald A, et al. BK virus: current understanding of pathogenicity and clinical disease in transplantation. Rev Med Virol 2019; 29: e2044. [DOI] [PubMed] [Google Scholar]
- 96. Goudsmit J, Wertheim-Van Dillen P, Van Strien A, et al. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol 1982; 10: 91–99. [DOI] [PubMed] [Google Scholar]
- 97. Cesaro S, Dalianis T, Rinaldo CH, et al. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother 2018; 73: 12–21. [DOI] [PubMed] [Google Scholar]
- 98. Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant 2008; 41: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Uhm J, Hamad N, Michelis FV, et al. The risk of polyomavirus BK-associated hemorrhagic cystitis after allogeneic hematopoietic SCT is associated with myeloablative conditioning, CMV viremia and severe acute GVHD. Bone Marrow Transplant 2014; 49: 1528–1534. [DOI] [PubMed] [Google Scholar]
- 100. Stracke S, Helmchen U, von Müller L, et al. Polyoma virus-associated interstitial nephritis in a patient with acute myeloic leukaemia and peripheral blood stem cell transplantation. Nephrol Dial Transplant 2003; 18: 2431–2433. [DOI] [PubMed] [Google Scholar]
- 101. Llewellyn MA, Gordon NS, Abbotts B, et al. Defining the frequency of human papillomavirus and polyomavirus infection in urothelial bladder tumours. Sci Rep 2018; 8: 11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gupta G, Kuppachi S, Kalil RS, et al. Treatment for presumed BK polyomavirus nephropathy and risk of urinary tract cancers among kidney transplant recipients in the United States. Am J Transplant 2018; 18: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cesaro S, Tridello G, Pillon M, et al. A prospective study on the predictive value of plasma BK virus-DNA load for hemorrhagic cystitis in pediatric patients after stem cell transplantation. J Pediatric Infect Dis Soc 2015; 4: 134–142. [DOI] [PubMed] [Google Scholar]
- 104. Varella RB, Zalona ACJ, Diaz NC, et al. BK polyomavirus genotypes Ia and Ib1 exhibit different biological properties in renal transplant recipients. Virus Res 2018; 243: 65–68. [DOI] [PubMed] [Google Scholar]
- 105. Special issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl. 3): S1–S155. [DOI] [PubMed] [Google Scholar]
- 106. Johnston O, Jaswal D, Gill JS, et al. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation 2010; 89: 1057–1070. [DOI] [PubMed] [Google Scholar]
- 107. Ito Y, Hino T, Honda A, et al. Fluoroquinolones for BK viral complication after transplantation: meta-analysis. Transpl Infect Dis 2020; 22: e13433. [DOI] [PubMed] [Google Scholar]
- 108. Mert D, Batgi H, Merdin A, et al. BK virus-associated hemorrhagıc cystitis in patients wıth allogeneıc hematopoıetıc cell transplantation: report of three cases. Hematol Rep. Epub ahead of print 26 June 2017. DOI: 10.4081/hr.2017.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen X-C, Liu T, Li J-J, et al. Efficacy and safety of leflunomide for the treatment of BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Acta Haematol 2013; 130: 52–56. [DOI] [PubMed] [Google Scholar]
- 110. Philippe M, Ranchon F, Gilis L, et al. Cidofovir in the treatment of BK virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2016; 22: 723–730. [DOI] [PubMed] [Google Scholar]
- 111. van Aalderen MC, Remmerswaal EBM, Heutinck KM, et al. Phenotypic and functional characterization of circulating polyomavirus BK VP1-specific CD8+ T cells in healthy adults. J Virol 2013; 87: 10263–10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Blyth E, Clancy L, Simms R, et al. BK virus-specific T cells for use in cellular therapy show specificity to multiple antigens and polyfunctional cytokine responses. Transplantation 2011; 92: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 113. Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther 2012; 20: 1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhou W, Sharma M, Martinez J, et al. Functional characterization of BK virus-specific CD4+ T cells with cytotoxic potential in seropositive adults. Viral Immunol 2007; 20: 379–388. [DOI] [PubMed] [Google Scholar]
- 115. Walls T, Shankar AG, Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis 2003; 3: 79–86. [DOI] [PubMed] [Google Scholar]
- 116. Houghton J, Li H, Fan X, et al. Mutations in bone marrow-derived stromal stem cells unmask latent malignancy. Stem Cells Dev 2010; 19: 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Humar A. Reactivation of viruses in solid organ transplant patients receiving cytomegalovirus prophylaxis. Transplantation 2006; 82: S9–S14. [DOI] [PubMed] [Google Scholar]
- 118. Fox JP, Hall CE, Cooney MK. The seattle virus watch. Am J Epidemiol 1977; 105: 362–386. [DOI] [PubMed] [Google Scholar]
- 119. CDC. Two fatal cases of adenovirus-related illness in previously healthy young adults — Illinois, 2000, https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5026a1.htm (accessed 3 January 2021). [PubMed]
- 120. Michaels MG, Green M, Wald ER, et al. Adenovirus infection in pediatric liver transplant recipients. J Infect Dis 1992; 165: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Norris SH, Butler TC, Glass N, et al. Fatal hepatic necrosis caused by disseminated type 5 adenovirus infection in a renal transplant recipient. Am J Nephrol 1989; 9: 101–105. [DOI] [PubMed] [Google Scholar]
- 122. Schwartz KL, Richardson SE, MacGregor D, et al. Adenovirus-associated central nervous system disease in children. J Pediatr 2019; 205: 130–137. [DOI] [PubMed] [Google Scholar]
- 123. Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 2003; 42: 466–472. [DOI] [PubMed] [Google Scholar]
- 124. Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 1998; 27: 1194–1200. [DOI] [PubMed] [Google Scholar]
- 125. Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis 2006; 43: 331–339. [DOI] [PubMed] [Google Scholar]
- 126. Flomenberg P, Babbitt J, Drobyski WR, et al. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis 1994; 169: 775–781. [DOI] [PubMed] [Google Scholar]
- 127. Shields AF, Hackman RC, Fife KH, et al. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med 1985; 312: 529–533. [DOI] [PubMed] [Google Scholar]
- 128. Emovon OE, Lin A, Howell DN, et al. Refractory adenovirus infection after simultaneous kidney-pancreas transplantation: successful treatment with intravenous ribavirin and pooled human intravenous immunoglobulin. Nephrol Dial Transplant 2003; 18: 2436–2438. [DOI] [PubMed] [Google Scholar]
- 129. Vyas JM, Marasco WA. Fatal fulminant hepatic failure from adenovirus in allogeneic bone marrow transplant patients. Case Rep Infect Dis 2012; 2012: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010; 116: 5476–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Koneru B, Atchison R, Jaffe R, et al. Serological studies of adenoviral hepatitis following pediatric liver transplantation. Transplant Proc 1990; 22: 1547–1548. [PMC free article] [PubMed] [Google Scholar]
- 132. Lee YJ, Chung D, Xiao K, et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant 2013; 19: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Robin M, Marque-Juillet S, Scieux C, et al. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica 2007; 92: 1254–1257. [DOI] [PubMed] [Google Scholar]
- 134. Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 2002; 100: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 135. La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis 2001; 32: 871–876. [DOI] [PubMed] [Google Scholar]
- 136. Myers GD, Krance RA, Weiss H, et al. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant 2005; 36: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 137. George D, El-Mallawany NK, Jin Z, et al. Adenovirus infection in paediatric allogeneic stem cell transplantation recipients is a major independent factor for significantly increasing the risk of treatment related mortality. Br J Haematol 2012; 156: 99–108. [DOI] [PubMed] [Google Scholar]
- 138. Hubmann M, Fritsch S, Zoellner AK, et al. Occurrence, risk factors and outcome of adenovirus infection in adult recipients of allogeneic hematopoietic stem cell transplantation. J Clin Virol 2016; 82: 33–40. [DOI] [PubMed] [Google Scholar]
- 139. Howard DS, Phillips GL, Reece DE, et al. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 1999; 29: 1494–1501. [DOI] [PubMed] [Google Scholar]
- 140. Allard A, Albinsson B, Wadell G. Detection of adenoviruses in stools from healthy persons and patients with diarrhea by two-step polymerase chain reaction. J Med Virol 1992; 37: 149–157. [DOI] [PubMed] [Google Scholar]
- 141. Lankester AC, van Tol MJD, Claas ECJ, et al. Quantification of adenovirus DNA in plasma for management of infection in stem cell graft recipients. Clin Infect Dis 2002; 34: 864–867. [DOI] [PubMed] [Google Scholar]
- 142. Leruez-Ville M, Minard V, Lacaille F, et al. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis 2004; 38: 45–52. [DOI] [PubMed] [Google Scholar]
- 143. Hiwarkar P, Kosulin K, Cesaro S, et al. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: state-of-the-art and real-life current approach. Rev Med Virol 2018; 28: e1980. [DOI] [PubMed] [Google Scholar]
- 144. Lion T, Kosulin K, Landlinger C, et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia 2010; 24: 706–714. [DOI] [PubMed] [Google Scholar]
- 145. Kosulin K, Berkowitsch B, Matthes S, et al. Intestinal adenovirus shedding before allogeneic stem cell transplantation is a risk factor for invasive infection post-transplant. EBioMedicine 2018; 28: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Campbell AP, Guthrie KA, Englund JA, et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis 2015; 61: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Roghmann M, Ball K, Erdman D, et al. Active surveillance for respiratory virus infections in adults who have undergone bone marrow and peripheral blood stem cell transplantation. Bone Marrow Transplant 2003; 32: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 148. Schilham MW, Claas EC, Van Zaane W, et al. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis 2002; 35: 526–532. [DOI] [PubMed] [Google Scholar]
- 149. Echavarria M, Herrera F, Solimano J, et al. Cyclic recovery of adenovirus in a stem cell transplant recipient: an inverse association with graft-versus-host disease. Bone Marrow Transplant 2003; 31: 301–303. [DOI] [PubMed] [Google Scholar]
- 150. César Pereira Santos H, Nunes Vieira Almeida T, Souza Fiaccadori F, et al. Adenovirus infection among allogeneic stem cell transplant recipients. J Med Virol 2017; 89: 298–303. [DOI] [PubMed] [Google Scholar]
- 151. Childs R, Sanchez C, Engler H, et al. High incidence of adeno- and polyomavirus-induced hemorrhagic cystitis in bone marrow allotransplantation for hematological malignancy following T cell depletion and cyclosporine. Bone Marrow Transplant 1998; 22: 889–893. [DOI] [PubMed] [Google Scholar]
- 152. Heemskerk B, Lankester AC, Van Vreeswijk T, et al. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J Infect Dis 2005; 191: 520–530. [DOI] [PubMed] [Google Scholar]
- 153. Hromas R, Cornetta K, Srour E, et al. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood 1994; 84: 1689–1690. [PubMed] [Google Scholar]
- 154. Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 2006; 12: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 155. Geyeregger R, Freimüller C, Stemberger J, et al. First-in-man clinical results with Good Manufacturing Practice (GMP)-compliant polypeptide-expanded adenovirus-specific T cells after haploidentical hematopoietic stem cell transplantation. J Immunother 2014; 37: 245–249. [DOI] [PubMed] [Google Scholar]
- 156. Metzgar D, Osuna M, Kajon AE, et al. Abrupt emergence of diverse of species B adenoviruses at US military recruit training centers. J Infect Dis 2007; 196: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 157. Radin JM, Hawksworth AW, Blair PJ, et al. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin Infect Dis 2014; 59: 962–968. [DOI] [PubMed] [Google Scholar]
- 158. Rubin LG, Levin MJ, Ljungman P, et al. Executive summary: 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58: 309–318. [DOI] [PubMed] [Google Scholar]
- 159. Van Montfrans J, Schulz L, Versluys B, et al. Viral PCR positivity in stool before allogeneic hematopoietic cell transplantation is strongly associated with acute intestinal graft-versus-host disease. Biol Blood Marrow Transplant. Epub ahead of print 15 January 2015. DOI: 10.1016/j.bbmt.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 160. PubMed. Cidofovir recommended for approval for CMV retinitis, https://pubmed.ncbi.nlm.nih.gov/11363308/ (accessed 3 January 2021). [PubMed]
- 161. Muller WJ, Levin MJ, Shin YK, et al. Clinical and in vitro evaluation of cidofovir for treatment of adenovirus infection in pediatric hematopoietic stem cell transplant recipients. Clin Infect Dis 2005; 41: 1812–1816. [DOI] [PubMed] [Google Scholar]
- 162. Hoffman JA, Shah AJ, Ross LA, et al. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7: 388–394. [DOI] [PubMed] [Google Scholar]
- 163. Ribaud P, Scieux C, Freymuth F, et al. Successful treatment of adenovirus disease with intravenous cidofovir in an unrelated stem-cell transplant recipient. Clin Infect Dis 1999; 28: 690–691. [DOI] [PubMed] [Google Scholar]
- 164. Neofytos D, Ojha A, Mookerjee B, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant 2007; 13: 74–81. [DOI] [PubMed] [Google Scholar]
- 165. Doan ML, Mallory GB, Kaplan SL, et al. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant 2007; 26: 883–889. [DOI] [PubMed] [Google Scholar]
- 166. Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the infectious diseases working party of the European group for blood and marrow transplantation. Bone Marrow Transplant 2003; 31: 481–486. [DOI] [PubMed] [Google Scholar]
- 167. Yaghobi R, Langari MJ, Ramzi M, et al. Molecular impact and inducible factors associated with adenovirus infection in hematopoietic skin cell transplant patients. Transplant Proc 2011; 43: 644–646. [DOI] [PubMed] [Google Scholar]
- 168. Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant 2012; 18: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Grimley MS, Chemaly RF, Englund JA, et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant 2017; 23: 512–521. [DOI] [PubMed] [Google Scholar]
- 170. Ciesla SL, Trahan J, Wan WB, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res 2003; 59: 163–171. [DOI] [PubMed] [Google Scholar]
- 171. Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol 2006; 134: 64–76. [DOI] [PubMed] [Google Scholar]