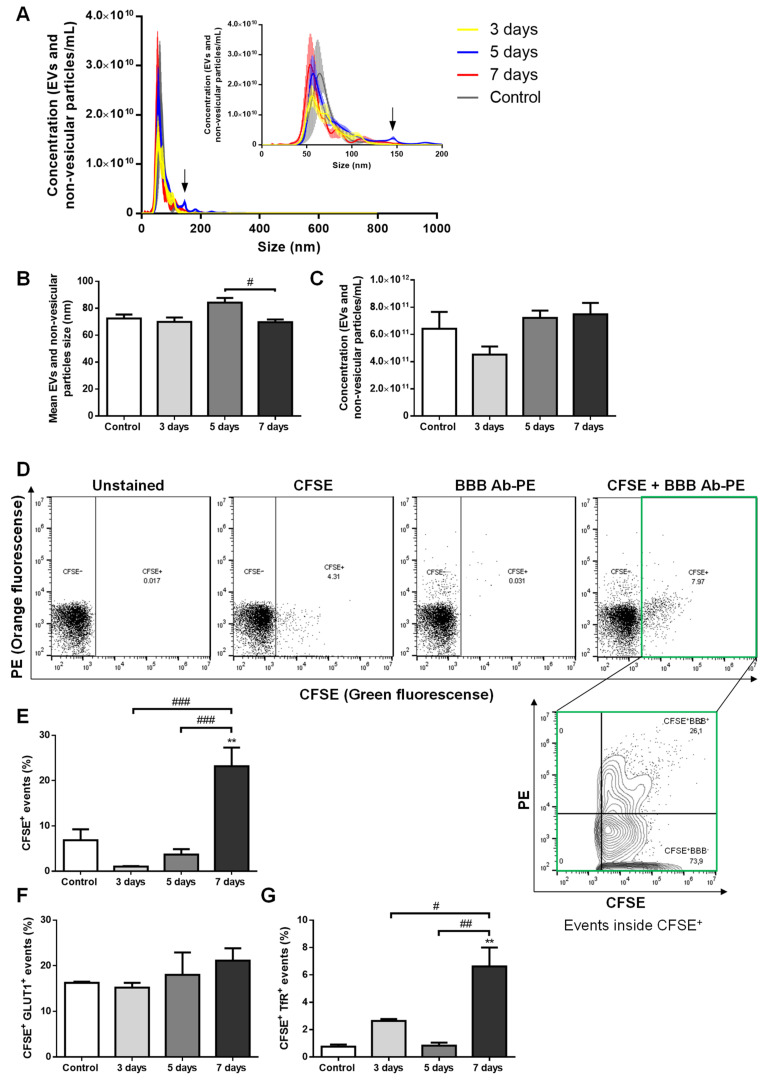
Figure 1.
Extracellular vesicles (EVs) are released by brain microvascular endothelial cells in advanced stages of breast cancer brain metastases (BCBM) formation. 4T1 cells or vehicle (control) were inoculated into the carotid artery of female Balb/c mice, whole blood was collected upon sacrifice after 3, 5 and 7 days, and plasma was processed for analysis of EVs (comprising exosomes and microvesicles/microparticles) and non-vesicular particles. Nanoparticle tracking analysis (NTA) was performed to assess size distribution (A), mean size (B) and concentration (C), which revealed a sustained presence of exosome-like EVs and non-vesicular particles with BCBM formation, with an additional tiny peak observed at 5 days (arrow in A). EVs of brain endothelial origin were determined by flow cytometry using carboxyfluorescein diacetate succinimidyl ester (CFSE) to detect vesicular particles (CFSE+) and phycoerythin (PE)-conjugated antibodies against the blood-brain barrier (BBB) markers, glucose transporter 1 (GLUT1-PE) and transferrin receptor (TfR-PE), collectively referred to as BBB Ab-PE. Representative plots of unstained, only CFSE-labelled, only BBB Ab-PE-labelled, and CFSE+BBB Ab-PE-labelled plasma samples are presented, which allowed the quantification of vesicular particles (CFSE+) of brain endothelial origin (BBB+) inside the CFSE+ population (CFSE+ BBB+) (D). The percentage of vesicular particle (CFSE+) events increased at 7 days after 4T1 cells injection (E). No statistically significant differences were observed in CFSE+ GLUT1+ events within the CFSE+ population with BCBM (F), while CFSE+ TfR+ events within the CFSE+ population increased at 7 days (G). Statistical differences are denoted as ** p < 0.01 vs. control, and as # p < 0.05, ## p < 0.01 and ### p < 0.001 between indicated conditions by One-way ANOVA. Data represented are means ± SEM, n = 3.