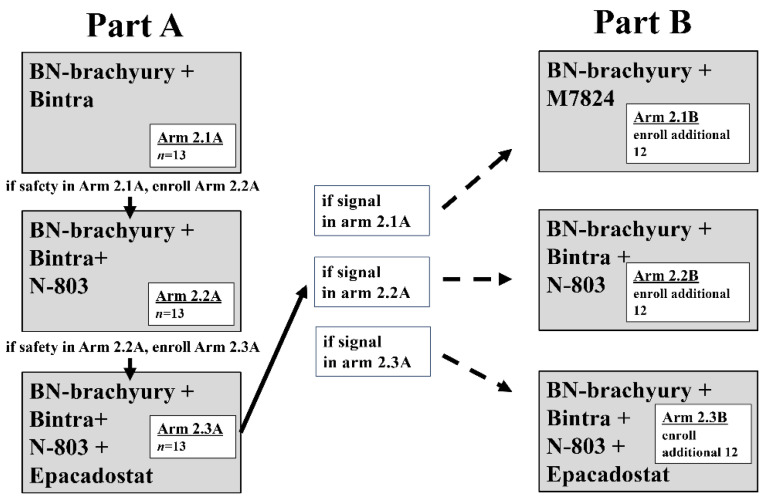
Figure 1.
Quick efficacy seeking trial design trial schema. During part A, enrollment to arms 1.1 and 2.1A begins simultaneously. Arm 1.1 is a dose-finding arm for N-803 combined with Bintrafusp alfa (Bintra), open to all solid tumors. After arm 2.1A completes accrual and safety of the combination has been demonstrated, and N-803 dosing has been determined from arm 1.1, arm 2.2A begins accrual. After arm 2.2A completes accrual and safety of the combination has been demonstrated, enrollment to arm 2.3A begins. Each of the 3 arms enrolls a total of 13 patients during part A. At the completion of part A, if there is a positive safety signal and a positive efficacy signal in arm 2.1A, 2.2A, or 2.3A, part B will begin. To further assess efficacy, arms in which an activity signal was observed (arms 2.1B, 2.2B, and/or 2.3B) may expand to a total of 25 patients. During part B, patients are randomized among all open arms to avoid selection bias.