Figure 1. Flow of Participants in a Study of the Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults.
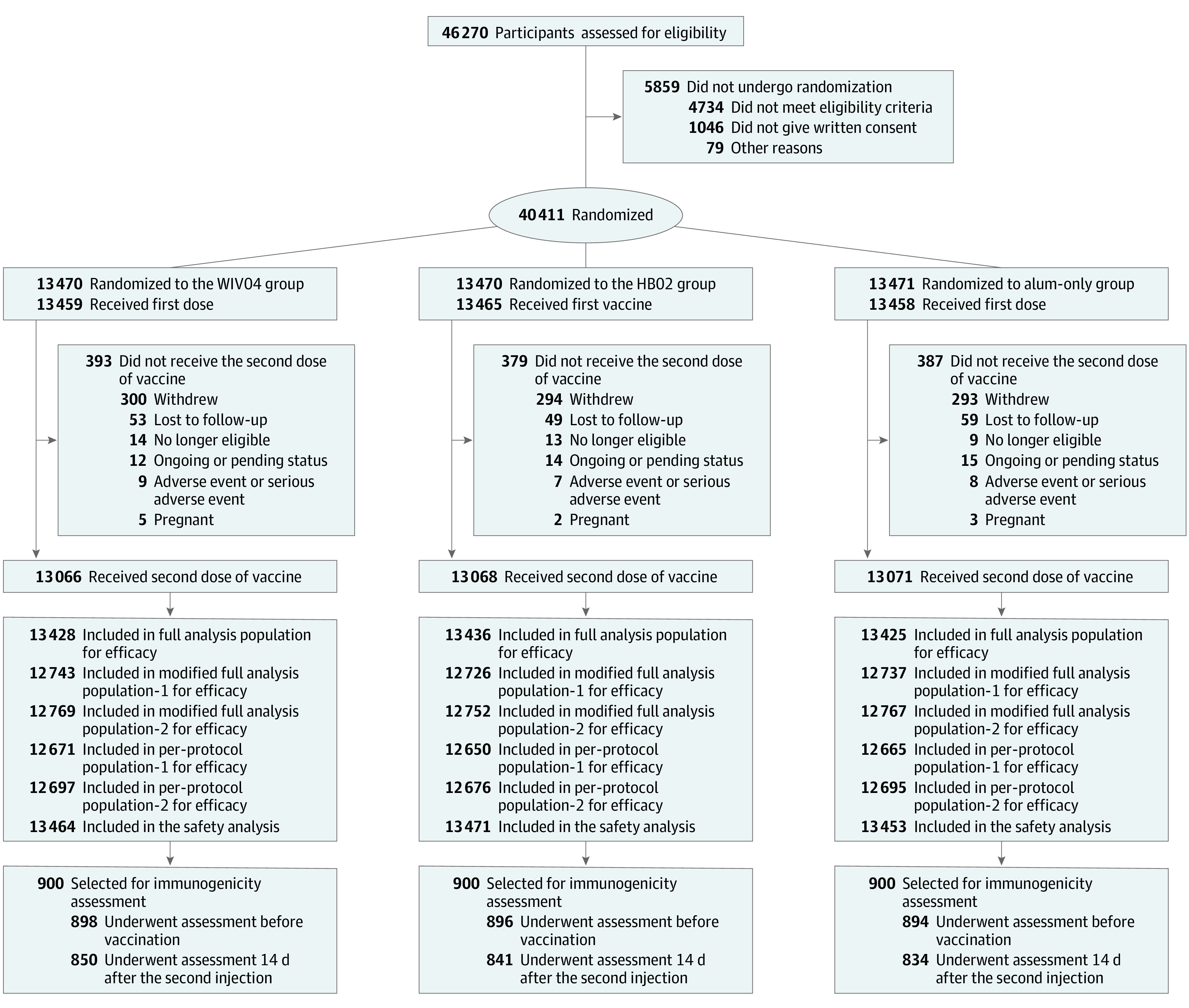
See eTable 1 in Supplement 2 for definitions of each analysis population. Among those receiving aluminum hydroxide (alum) for the first dose, 5 participants received WIV04 (n = 2) and HB02 (n = 3) vaccines for the second dose and were not included in the safety analysis population of the alum-only group (13 458 – 5 = 13 453), but were included in the WIV04 and HB02 groups, respectively. Three participants who received WIV04 for the first dose and HB02 for the second dose and 3 participants who received HB02 for the first dose and WIV04 for the second dose were included in both groups (WIV04: 13 459 + 2 + 3 = 13 464; HB02: 13 465 + 3 + 3 = 13 471). To measure neutralization antibody levels, the first 900 participants from each study site were selected. There were 9 participants in the WIV04 group, 7 in the HB02 group, and 8 in alum-only group who had adverse events or serious adverse events (SAEs) after the first dose and did not receive the second dose, and only 1 SAE in the HB02 group was deemed to be vaccine-related. See eTables 7 and 8 in Supplement 3 for a list of serious adverse events.
