Figure 3. Common Adverse Reactions and Grades Within 7 Days After 2 Doses in the Safety Analysis Set.
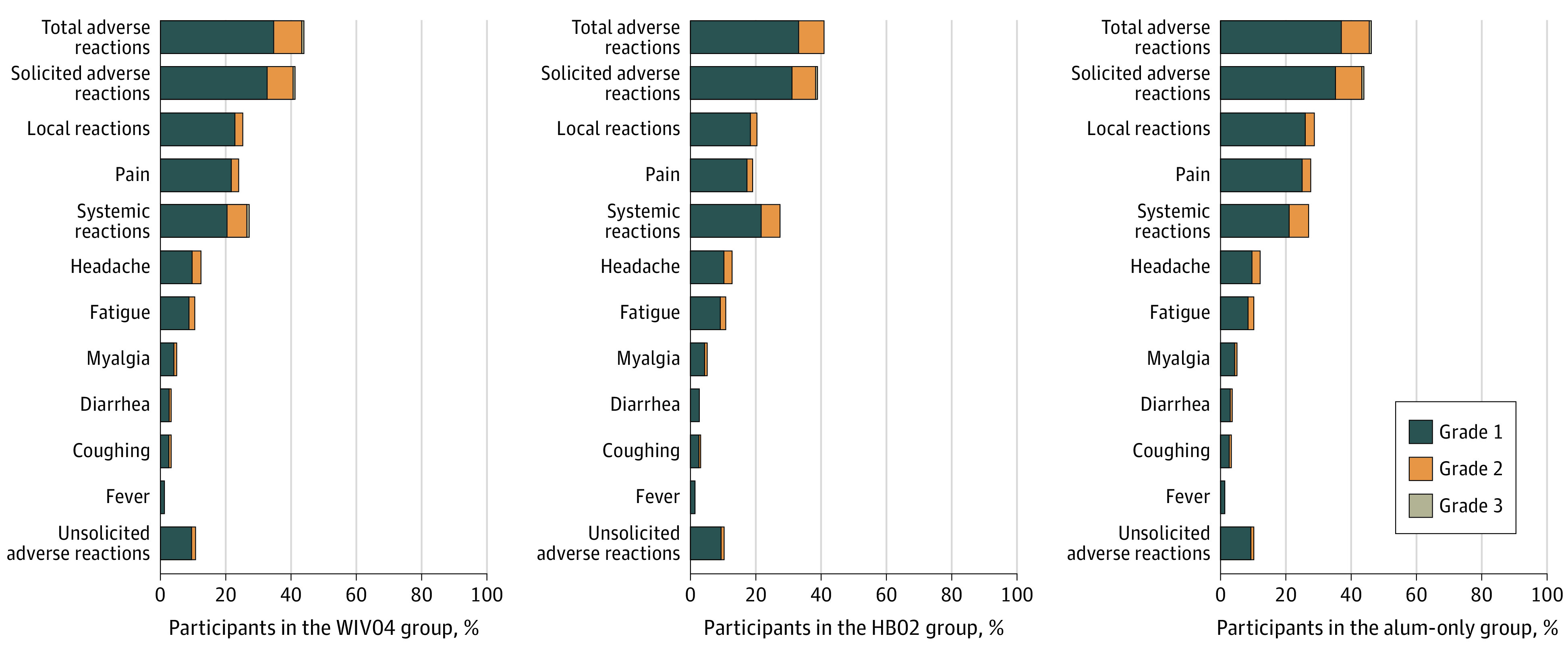
The safety analysis population included all participants who received at least 1 dose. Only adverse reactions that occurred in at least 2% of participants are included; see eTable 5 in Supplement 2 for details of all adverse reactions (including common and less common adverse reactions). Participants with more than 1 adverse reaction in a specific reaction category were only counted once; for example, if they had the same symptom (eg, injection-site pain) after each dose or if they had more than 1 symptom in the reaction class (total, systemic, and local), they were only counted once in that adverse reaction class. Participants with both lower- and higher-grade adverse events were counted once in the higher-grade total adverse events. Grading scales for systemic and local adverse events are detailed in the protocol in Supplement 1. Alum indicates aluminum hydroxide.
