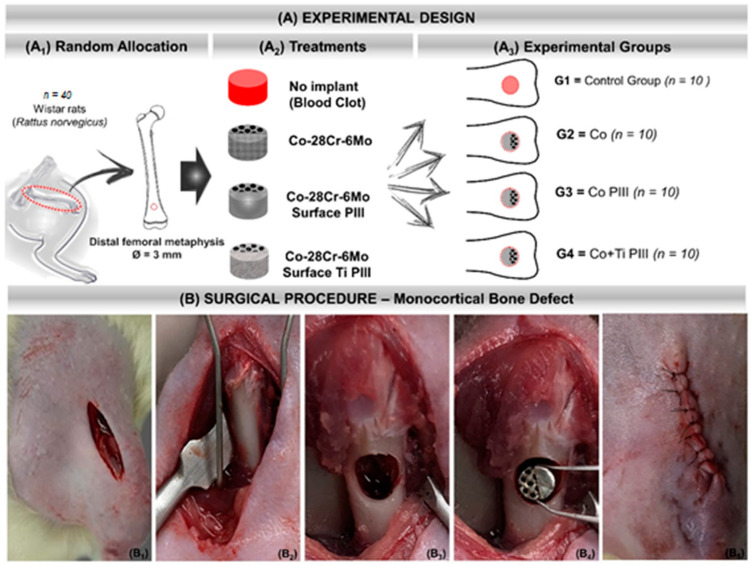
Figure 1.
(A) Experimental design. (A1) Random Allocation—Forty adult male Wistar rats (Rattus norvegicus), 120 days old, weighing around 340 g. Monocortical bone defect model 3 mm in diameter in the distal metaphysis of the left femur. (A2) Treatments—groups according to the type of implant or not: No implant (blood clot), Co-28Cr-6Mo, Co-28Cr-6Mo with surface nanostructured by PIII and Co-28Cr-6Mo with surface coated with Ti and nanostructured by PIII. (A3) Experimental groups: G1 (n = 10)—bone defect filled with blood clot, no implant; G2 (n = 10)—bone defect filled with Co-28Cr-6Mo implant; G3 (n = 10)—bone defect filled with Co-28Cr-6Mo implant with surface nanostructured by PIII; G4 (n = 10)—bone defect filled with Co-28Cr-6Mo implant with surface coated with Ti and nanostructured by PIII. (B) Surgical procedure. (B1) Skin incision on the anteromedial aspect of the thigh. (B2) Exposure of the cortical bone of the surgical area. (B3) 3 mm bone defect in the distal metaphysis of the femur. (B4) Implantation of the metallic alloy in the surgical bed. (B5) Tegument suture with 5-0 nylon thread.