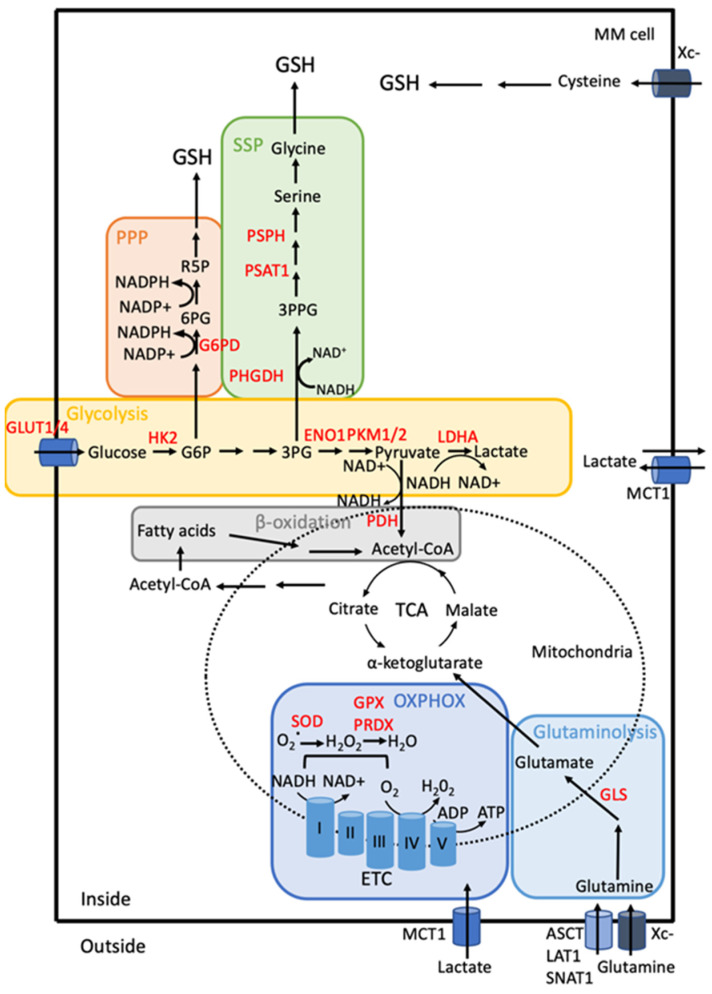
Figure 3.
Schematic representation of metabolism rewiring in MM cells. MM cells are glycolytic. GLUT1/4, glucose transporters. The levels of GLUT1/4 are high and allow an elevated glucose uptake. Intracellular glucose is transformed into lactate by oxidative glycolysis. This multi-step catabolism is controlled by several glycolytic enzymes all overexpressed in MM cells such as HK2, PFK, PKM2, LDHA [19,110,137,138]. The end-product of glycolysis, lactate is secreted by MM cells. However, MM cells take up exogenous lactate produced by the surrounding TME. Lactate enters cells through the MCT1/4 transporters and fuels OXPHOS fully performing in MM. Glutamine synthase is lacking in MM cells, therefore MM cells are dependent on glutamine uptake. By contrast, glutaminase (GLS) expression is increased [139]. PDH is a gatekeeper enzyme that converts pyruvate into acetyl-CoA and regulates the mitochondrial metabolism. PDK1 is a serine/threonine kinase that negatively regulates PDH, its inhibition induces MM cell death [133]. The serine metabolism is also involved in MM cell adaptation to BTZ treatment. Phosphoglycerate dehydrogenase, PHGDH, the first limiting enzyme of the SSP pathway that converts 3-PG into PHP is elevated and increases GSH synthesis in BTZ-resistant cells [20,137]. G6PD, the first rate-limiting enzyme of the PPP, converting G6P into 6PG, is elevated in MM cells and associated with an exacerbated flux [140]. Associated with increased mitochondrial biomass and function, MM cells have higher concentrations of ATP, NADH/NADPH ratio and mitochondrial membranes are hyperpolarized, all these changes leading to impaired drug response and resistance [129,141]. β-oxidation participates in the myeloma metabolism changes [19]. The abbreviations used in the Figure 3 are gathered at the end of the text.