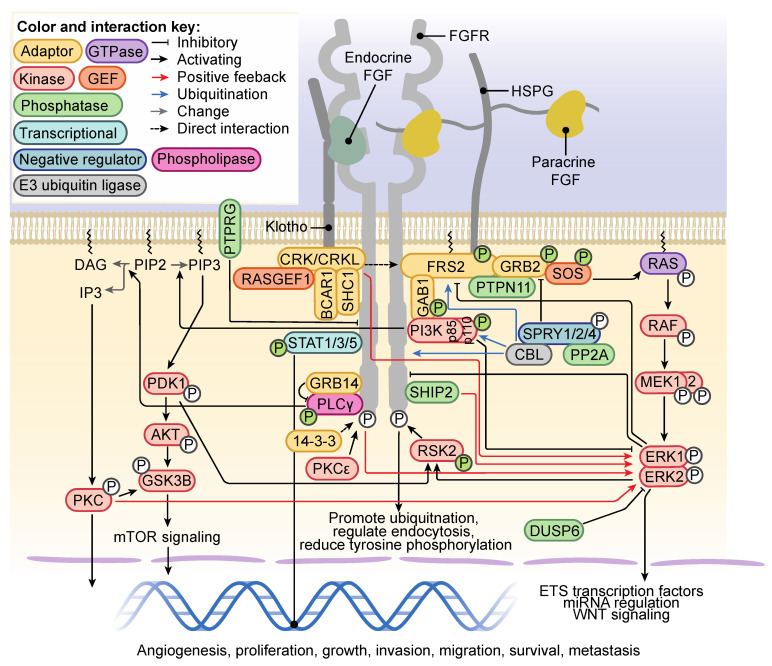
Figure 2.
Fibroblast Growth Factor Receptor (FGFR) signaling partners, pathways and regulation of FGFR signaling Klotho or heparan sulfate proteoglycan (HSPG) are required for activation of FGFR by endocrine or paracrine FGFs, respectively. Binding of the ligand initiates large-scale phosphorylation and activation of intracellular signaling cascades. RAS-mitogen activated protein kinase (MAPK) and phosphatidylinositide 3-kinase (PI3K)-AKT signaling are dependent on FRS2 binding FGFR, which is associated with a CRK/CRKL complex to positively regulate extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling. ERK1/2 signaling is tightly regulated downstream of FGFR. The PI3K p85 subunit can bind FGFR independently of growth factor receptor bound 2 (GRB2)-associated binding protein 1 (GAB1) to activate AKT signaling through conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). PI3K-AKT signaling activates the mammalian target of rapamycin (mTOR) signaling downstream FGFR activation. PLCγ binds FGFR and hydrolyses PIP2 to produce diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 activates protein kinase C (PKC) signaling. STAT1/3/5 activation by FGFR results in translocation to the nucleus to regulate transcription. Positive regulators of FGFR signaling include 14-3-3, protein kinase C ε (PKCε) and PIP3 5-phosphatase 2 (SHIP2), whereas negative regulators include SPROUTY 1/2/4 (SPRY1/2/4), E3-ubiquitin ligase CBL (CBL), protein phosphatase 2 A (PP2A), dual specificity phosphatase 6 (DUSP6) and protein tyrosine phosphatase receptor type G (PTPRG). Phosphorylation colored green indicates proteins where activation is dependent on FGFR-mediated tyrosine phosphorylation.