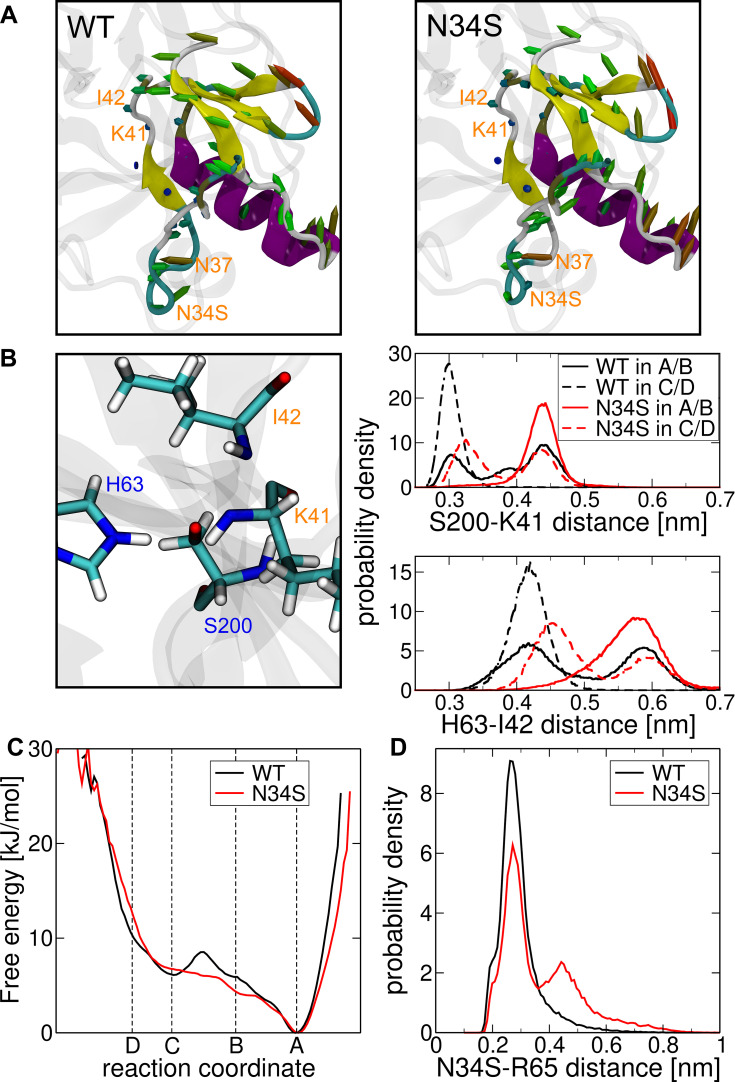
Figure 3.
(A) Porcupine plots that show the structural difference when state II A/B is transitioned to state II C/D for the mutant right and WT left (cf. Figures 1 and 2A). The colors indicate the secondary structure elements for helix purple, sheet yellow, turns cyan and coil white. The marked arrows indicate the transposition of the Cα atoms with the color gradient blue-green-red for small-medium-large deviations, respectively. (B) Distance distributions in the active center between the hydroxy oxygen atom of S200 and the carboxamide carbon atom of K41 (top), and between the far ring hydrogen of H63 and carboxamide nitrogen atom of I42 (bottom). The distributions are measured from respective REMD simulations in state II A/B and state II C/D of the mutant (N34S) and wild type (WT). (C) Free energy in kJ/mol along the reaction coordinate determined by transition path sampling of the wild-type solid and mutant dashed. (D) Histogram of the minimal distance between the amino acids N34S and R65 for wild-type black and mutant red.