Fig. 3. Mean change from baseline in ALP over time by UDCA baseline use.
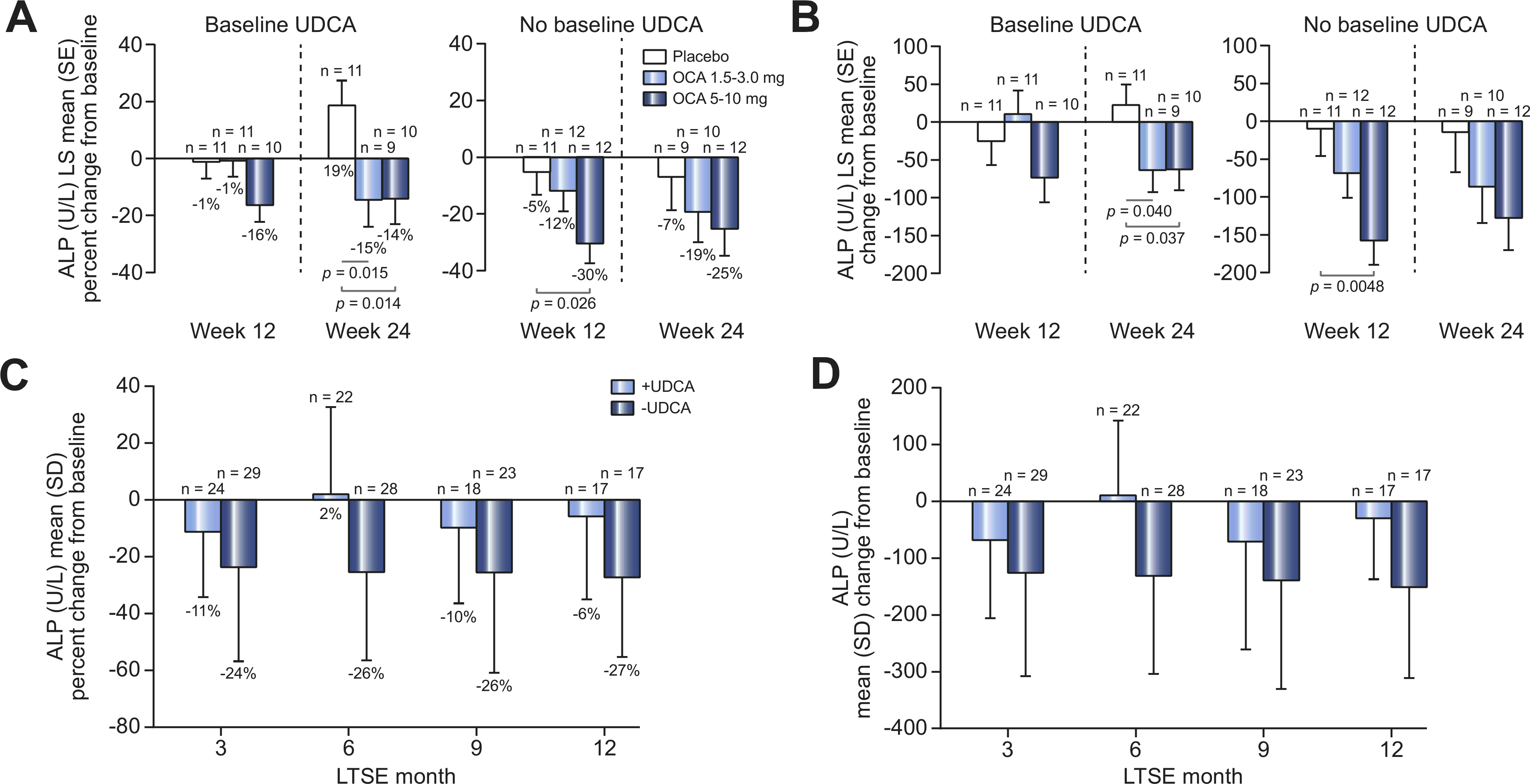
(A) Percentage or (B) absolute LS mean (SE) change from baseline in serum ALP (U/L) by UDCA baseline use in the DB phase (safety population). Mean baseline ALP (U/L) (SEM) UDCA use: 485.8 (104.0) (placebo), 432.4 (36.5) (OCA 1.5–3.0 mg), 387.1 (33.3) (OCA 5–10 mg); no UDCA use: 642.2 (66.8) (placebo), 413.4 (34.5) (OCA 15–3.0 mg), 468.6 (54.1) (OCA 5–10 mg). (C) Percentage or (D) absolute mean (SD) change from baseline in serum ALP by UDCA baseline use for all patients in the LTSE (pooled data). Mean baseline ALP (U/L) (SD) UDCA use, 427.5 (249.6); no UDCA use, 482.9 (199.8). ANCOVA was used for these analyses. ALP, alkaline phosphatase; ANCOVA, analysis of covariance; DB, double-blind; LS, least-square; LTSE, long-term safety extension; OCA, obeticholic acid; UDCA, ursodeoxycholic acid.
