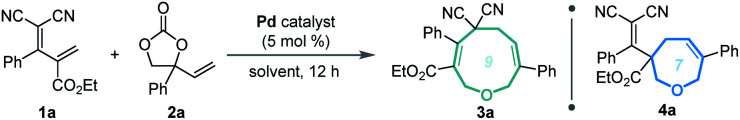
Optimization studies for the annulation of allylidenemalononitril 1a and VEC 2aa.
| |||||
|---|---|---|---|---|---|
| |||||
| Entry | Catalyst | Solvent | Temp. (°C) | Yieldb (%) | 3a : 4ac |
| 1d | Pd(PPh3)4 | Toluene | 20 | 72 | 3.5 : 1 |
| 2 e | Pd(PPh 3 ) 4 | MeCN | 20 | 96(90) | >20 : 1 |
| 3d | Pd(PPh3)4 | DCM | 20 | 68 | 3.6 : 1 |
| 4d | Pd(PPh3)4 | CHCl3 | 20 | 85 | 2.6 : 1 |
| 5 | Pd(PPh3)4 | THF | 20 | 85 | 1.4 : 1 |
| 6e | Pd(PPh3)4 | DMF | 20 | 16 | >20 : 1 |
| 7f | Pd/L1–L7 | THF | 20 | <5 | — |
| 8 | Pd(PPh3)4 | THF | 40 | 98 | 1 : 4.6 |
| 9 | Pd(PPh 3 ) 4 | THF | 60 | 91(84) | <1 : 20 |
| 10 | Pd(PPh3)4 | THF | 80 | 89 | <1 : 20 |
| 11 | Pd(PPh3)4 | 1,4-Dioxane | 60 | 83 | 16.0 : 1 |
| 12 | Pd(PPh3)4 | Toluene | 60 | 88 | 1 : 1.3 |
| 13 | Pd(PPh3)4 | MeCN | 60 | 87 | 8.6 : 1 |
| 14 | Pd(PPh3)4 | DMF | 60 | 81 | 14.8 : 1 |
| 15 | Pd(PPh3)4 | DCM | 60 | 76 | 1 : 1.4 |
| 16 | Pd(PPh3)4 | CHCl3 | 60 | 80 | 5.3 : 1 |
Unless noted otherwise, the reactions were carried out with 1a (0.10 mmol), 2a (0.15 mmol) and the Pd catalyst (5 mol%) in solvent (1 mL) for 12 h.
Yield was determined by 1H-NMR analysis with CH2Br2 as the internal standard; the data in parentheses refer to isolated yields.
The ratio of 3a : 4a was determined by 1H-NMR analysis of the crude reaction mixture.
For 48 h.
For 24 h.
The Pd/ligand complex was pre-prepared with Pd2(dba)3·CHCl3 and a ligand in THF at rt for 1 h.
