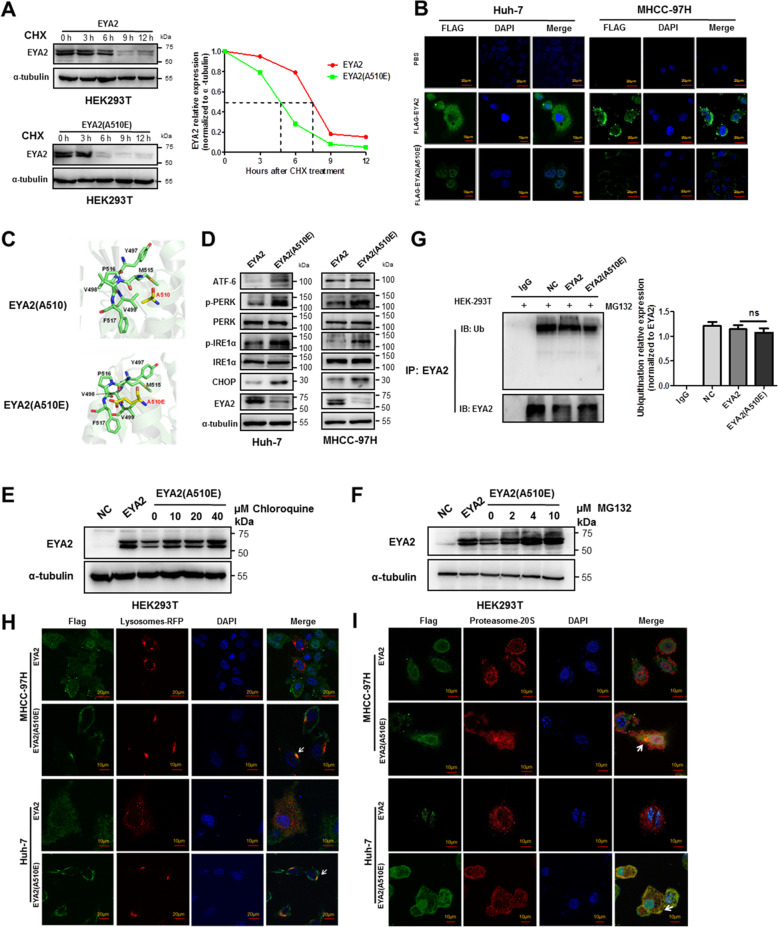
Fig. 5.
EYA2(A510E) mutation leads to lysosomal or proteasomal degradation by triggering the unfolded protein response. (A) EYA2(A510E) shortened the half-life of EYA2 protein. EYA2 and EYA2(A510E) stably transfected HEK293T cells were treated with 100 μg/ml cycloheximide (CHX) and protein lysates were collected at the indicated times for western blot analysis. (B) Confocal immunofluorescence analysis of the expression of Flag-tagged EYA2(A510E) and Flag-tagged EYA2. (C) Structural prediction of EYA2(A510E) mutation. (D) Western blot analysis of the expression of UPR-related proteins in EYA2(A510E) mutant-expressed Huh-7 and MHCC-97H cells. Western blot analysis of EYA2 expression in EYA2(A510E) stably transfected HEK293T cells treated with chloroquine (E) or MG132 (F). (G) Immunoprecipitation analysis of the ubiquitination of EYA2(A510E) mutation. Western blot densitometry was performed from three independent experiments. EYA2 was used as the normalization control. (H) Colocalization of Flag-tagged EYA2(A510E) and lysosomes in the presence of chloroquine detected by confocal immunofluorescence. The arrows point the colocalization of Flag-tagged EYA2(A510E) and Lysosomes-RFP. (I) Colocalization of Flag-tagged EYA2(A510E) and proteasome-20S in the presence of MG132 detected by confocal immunofluorescence. The arrows point the colocalization of Flag-tagged EYA2(A510E) and proteasome-20S. ns: not significant. (G) mean ± SEM, Student’s t-tests