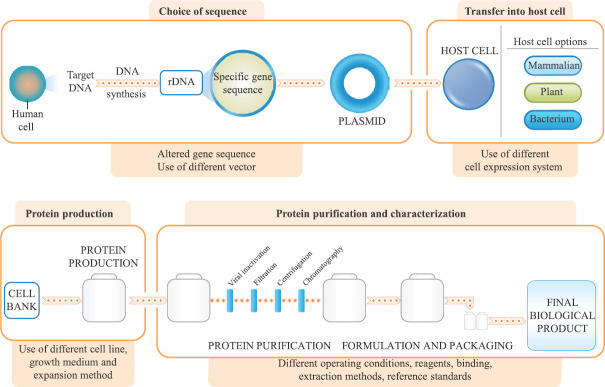
Fig. 3.
Variation between manufacturing of biologics and similar biologics. The manufacturing process to produce biologics and similar biologics, include complex and controlled procedures. The process involves cloning of the relevant gene into a DNA vector and transferring it into a host cell. After the protein expression, appropriate cell line is selected and expanded in a growth medium using suitable expansion method. Complex purification and validation procedures are followed to obtain the purified final biological product. The characteristics of the final product may differ based on variation in selection of the DNA sequence, cloning, transfection, amplification, purification, formulation and validation procedure followed. rDNA, recombinant DNA.