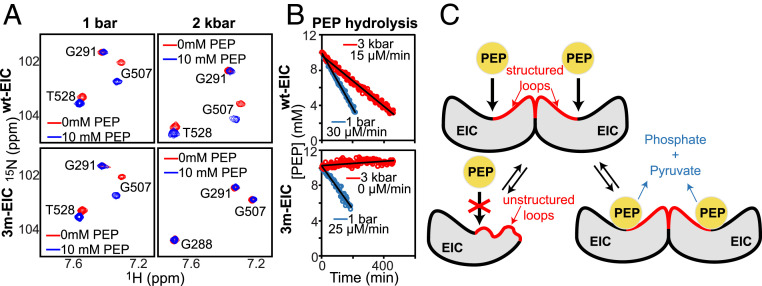
Fig. 3.
Monomeric EIC does not bind nor hydrolyze PEP. (A) A selected region of the 1H-15N TROSY spectrum of wt-EIC at 1bar (Top Left), wt-EIC at 2 kbar (Top Right), 3m-EIC at 1 bar (Bottom Left), and 3m-EIC at 2 kbar (Bottom Right) acquired in the absence (red) and in the presence (blue) of 10-mM PEP is shown. Note that monomerization of 3m-EIC at 2 kbar generates large shifts for T528 and G288 cross-peaks. (B) Rate of the PEP hydrolysis reaction catalyzed by 150-μM wt-EIC (Top) and 3m-EIC (Bottom) at 65 °C and an external pressure of 1 bar (blue) and 3 kbar (red). A pressure of 3 kbar was used to ensure that the monomer–dimer equilibrium of 3m-EIC is fully shifted toward the monomeric species. (C) Schematic representation of the effect of monomerization on the structure and biological activity of EIC. The catalytic loops α3β3, α6β6, and α7β7, which form a large portion of the dimer interface, are largely disordered in monomeric EIC and become ordered upon dimerization.