Figure 4.
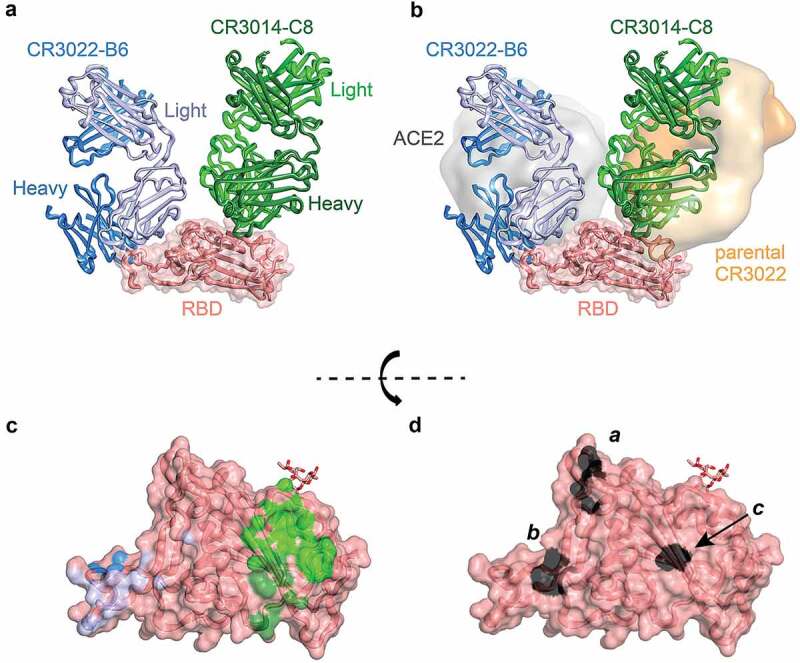
Crystal structure of CR3022-B6 and CR3014-C8 in complex with SARS-CoV-2 RBD. (a) CR3022-B6 (blue) and CR3014-C8 (green) Fabs bound to SARS-CoV-2 RBD (salmon); (b) CR3022-B6 binding to an RBD epitope overlapping with the ACE2 (gray surface, PDB entry 6m0j) interface and different to the parental CR3022 (orange surface, PDB entry 6w41) epitope; CR3014-C8 binding to an epitope distant from the ACE2 interface; (c) Antibody contact surfaces on RBD for CR3022-B6 (blue) and CR3014-C8 (green). The majority of the RBD surface is buried by antibody VL domains (light blue and light green), with more limited VH interactions (dark blue and dark green); (d) RBD surface with residues targeted for epitope mapping in black; surface a (T500, N501 and Y505) ACE2 binding interface; surface b (L455 and F456) CR3022-B6 and CR3014-D1 interface (adjacent to the ACE2 binding site); surface c (K378) parental CR3022 binding interface
