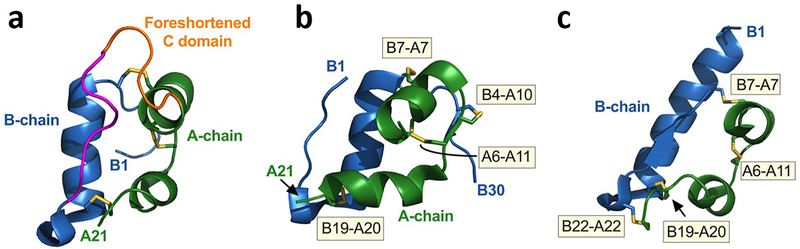
Fig. 2.
Ultra-stable insulin analogues. (a) Single-chain insulin (SCI) analogues exhibit native-like A and B domains (green and blue, respectively) with three native disulfide bridges (yellow). A foreshortened C-domain (5–8 residues; orange) connects the C-terminal B-chain β-strand (magenta) to the A-chain N-terminus. A favourable C-domain sequence can dampen conformational flexibility, augment thermodynamic stability and protect from fibrillation [66]. Protein Data Bank (PDB) ID: 2LWZ (www.rcsb.org/; accessed: 1 February 2021). Figure adapted from [145] with permission from Wolters Kluwer Health, Inc. (b, c) Ultra-stable two-chain insulin analogues with engineered fourth disulfide bridges. Crystal structure of the four-disulfide (4SS)-insulin analogues containing a disulfide bond in (b) position A10-B4 (PDB ID: 4EFX) [67] and (c) position A22-B22 (PDB ID: 6TYH) [69]. The disulfide bonds (shown in yellow) and labelled (yellow boxes). Images were created with PyMOL (https://pymol.org/2/; accessed: 1 February 2021).