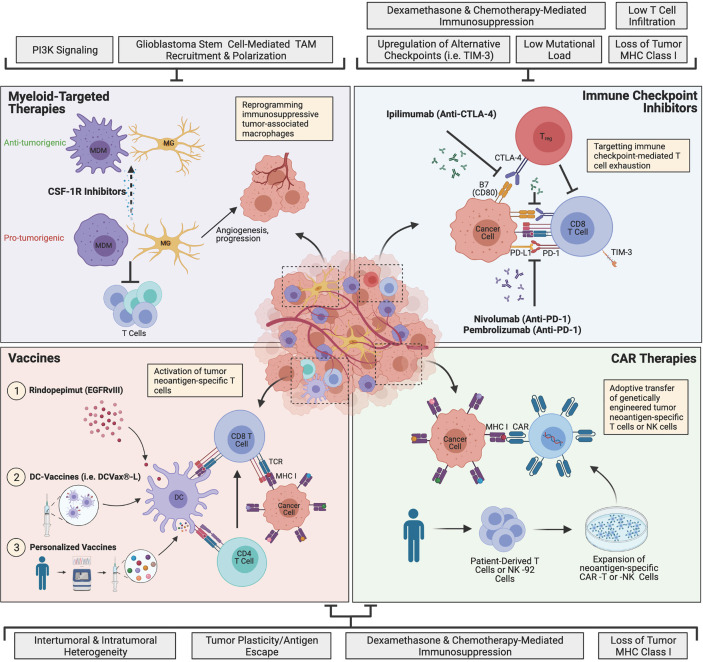
Figure 1.
The current landscape of major glioblastoma immunotherapies and mechanisms of resistance. Immune checkpoint inhibitors (ICIs) target T cell exhaustion through blockade of immune checkpoints PD-1 and CTLA-4 to restore T cell function and antitumor activity. Myeloid-targeted therapies such as CSF-1R inhibitors reprogram immunosuppressive microglia (MG) or monocyte-derived macrophages (MDMs) (pro-tumorigenic) to become more anti-tumorigenic. Peptide vaccines, dendritic cell (DC)-vaccines and personalized vaccines educate T cells to target tumor neoantigen(s). Chimeric antigen receptor (CAR) immunotherapies involve genetically engineering a patient’s own T cells or non-patient NK-92 cells to express neoantigen-specific CARs, which are expanded in culture and adoptively transferred to the patient. Glioblastoma is highly resistant to therapy, and currently, none of the depicted immunotherapies have succeeded in improving treatment, although many clinical trials are currently ongoing. The grey boxes outline major mechanisms of resistance that are barriers to each immunotherapeutic approach, including intrinsic, adaptive and iatrogenic mechanisms. Image made with BioRender.com.