Table 2. Clinical impact of considering non-Mendelian models of DCM genetic architecture.
Broadening the scope of the genetic evaluation of DCM to consider multi-variant and gene-environment interaction models of disease has downstream implications for the approach to risk assessment as well as the ongoing management and care of DCM patients and families. However, any recommendations beyond those guideline-based are recommended to be conducted within an investigational environment, as mechanisms to define moderate-impact gene-disease relationships, to adjudicate disease-contributing variants that exceed a monogenic model, and to quantify environmental contributions to disease, in a clinical setting are needed to provide a tailored approach to care.
| Models of DCM Genetic Architecture | |||
|---|---|---|---|
| Components of a Genetic Evaluation | Mendelian Model | Multivariant Model | Gene-Environment Interaction (GxE) Model |
| Proband Medical History | • Exclude all other known clinical causes of DCM. | • Exclude all other known clinical causes of DCM. | • Comprehensive evaluation of environmental factors that may be contributing to DCM phenotype. |
| Family History (FHx) | • Identify a single lineage harboring the genetic risk. • ≥3 generations of FHx.57 |
• Seek evidence of bilineal inheritance. • Spectrum of penetrance and expression likely. • ≥3 generations of FHx. |
• Seek evidence of bilineal inheritance. • Comprehensive clinical and environmental data. • Spectrum of penetrance and expression expected. • >3 generations of FHx. |
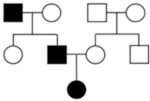
| Pedigree Considerations | • Dominant features (e.g. males and females affected, male-to-male transmission, multiple generations with disease). • Variable expression and reduced penetrance may complicate interpretation. |
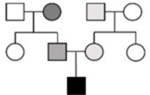
• May appear de novo or recessive, as proband may be the only individual with a complete DCM phenotype arising from a unique variant burden. • Variable age of onset may be observed depending on variant burden of each individual. • Relatives may have subtle/mild disease, be asymptomatic, or unaffected. |
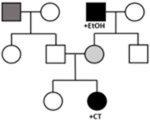
• May appear de novo or recessive, as the proband may be the only individual with a complete DCM phenotype with sufficient burden of genetic and environmental factors • Variable age of onset as environmental factors more likely to occur in adulthood and multiple factors required to meet disease threshold • Relatives may have subtle/mild disease, be asymptomatic, or unaffected |
Pedigree Samples
|
 |
 |
 |
| Risk Counseling | • 50% chance to FDRs to share genetic predisposition. • Individuals with genetic predisposition may not develop disease at the same age or severity. |
• Discussion of disease threshold model, where multiple variants additively cause the phenotype. • 50% chance to FDRs to have each individual variant. • Reduced penetrance driven by variant burden. • Relatives may have mild or subclinical disease or be unaffected. |
• Discussion of disease threshold model, where multiple variants interact with environmental factor(s) to cause the phenotype. • 50% chance to FDRs to have each individual variant • Relatives may have mild or subclinical disease or be unaffected. • Environmental factors can change over time (e.g. age) • Motivational counseling strategies important in order to inspire preventive health behaviors, emphasizing on the role of environment and managing non-genetic risk factors as possible to mitigate risk. |
| Clinical Evaluation of Family | • ECHO or CMR, and ECG, of FDRs at baseline and repeated at age-defined intervals.28 | • ECHO or CMR, and ECG, of FDRs at baseline. • Above repeated at frequency recommended by clinician based on genetics and family presentation until data-driven guidelines available. |
• ECHO or CMR, and ECG, of FDRs at baseline. • Above repeated at frequency recommended by clinician based on genetics, environmental factors, and family presentation until data-driven guidelines available. |
| Genetic Testing (GT) of Proband | • 19 high evidence genes.5 | • At least 51 genes with varying degrees of human genetic evidence.5 • Engage in research to investigate DCM genetic architecture. |
• At least 51 genes with varying degrees of human genetic evidence.5 • Engage in research to investigate DCM genetic architecture, which may include calculation of polygenic risk. |
| Variant Interpretation | • Very rare variants (MAF <0.01% in all non-founder subpopulations111). • Case-level criteria to only include counts for strictly applied DCM phenotype.6 |
• Very rare, rare and low frequency variants (MAFs <0.01%, <0.05%, and <1%) • Case-level criteria to include idiopathic DCM and incomplete DCM phenotypes in counts (e.g. rEF only, LVE only, CSD only, etc) |
• Rare to common variation. • Case-level criteria for rare variants to include broad inclusion of phenotypes in case counts, including incomplete DCM phenotypes and GxE DCM phenotypes. |
| GT Considerations for FDRs | • GT of FDRs if P/LP variant(s) found in proband. • Discharge those with negative cascade GT from follow up.28 |
• Consider FDR GT for disease-contributing/causing variants, regardless of Mendelian classification. | • Consider FDR GT for disease-causing/contributing variants, regardless of Mendelian classification • Quantification of environmental risk burden (when data-driven approach available). |
| Clinical Recommendations | • Communicate risk with family • Baseline clinical screening of FDRs per guidelines. • Cascade GT of FDRs when applicable. • Continued surveillance of FDRs with genetic risk per guidelines.28 |
• Communicate risk with family. • Clinical screening of FDRs per guidelines. |
• Communicate risk with family. • Clinical screening of FDRs per guidelines. |
| Research Recommendations | • Research participation to study penetrance, expression, and additional genetic architecture of Mendelian DCM • This may also include investigational approaches to care. |
• Research participation to investigate DCM genetic architecture, which may also include investigational approaches to care: ○ Consider cascade GT of FDRs, and possibly second-degree relatives as indicated by pedigree, when relevant disease-causing/contributing variants are identified in the proband. ○ Relatives discharged from continued surveillance if all relevant variants are absent and clinical screening is negative, informed by clinical judgement. |
• Research participation to investigate DCM genetic architecture, which may also include investigational approaches to care: ○ Consider cascade GT that may well exceed FDRs when relevant disease-causing/ contributing variants are identified in the proband. ○ Quantification of environmental risk. ○ Consider continuing clinical surveillance in relatives if GxE burden is estimated to approach the disease threshold. ○ If not initially estimated to have a GxE burden warranting ongoing surveillance, consider repeating environmental risk quantification to re-estimate environmental risk burden; if elevated, surveillance recommendations may change as informed by clinical judgement. |
