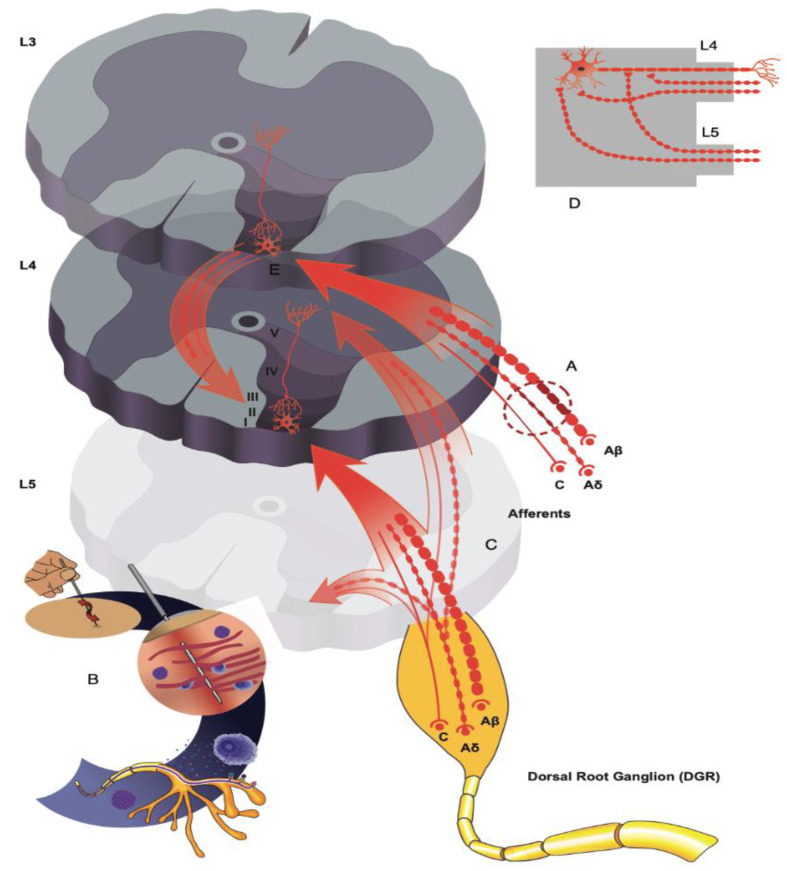
Figure 3.
Schematic illustration of neuropathic pain mechanisms and intersegmental needling. (A) Nerve injury downregulates MOR and KCC2 expression in the dorsal horn weakening segmental inhibition. (B) Needle insertion with retention or manipulation activates mechanosensitive channels on afferent fibers, MC and fibroblasts promoting release of ATP. ATP is broken down into adenosine to provide antinociceptive effects locally and at the spinal level by activating A1Rs on primary afferent terminals and interneurons in lamina Ⅱ. (C) Needle stimuli aimed at rostro-caudal segments away from the primary segment of nerve injury will activate Aδ and C fiber arborizations of neighboring roots that synapse with interneurons in lamina Ⅰ-Ⅱ at the segment of injury (D) Needle induced increases of GABA, NA and adenosine potentiate presynaptic and postsynaptic analgesic effects through volume transmission. (E) Needle stimuli will inhibit microglial activation leading to downregulation of the BDNF and TrkB pathway, increasing KCC2 expression in the lumbar dorsal horn at the segment of nerve injury, and restoring chloride regulation in dorsal horn neurons. MOR, -opioid receptor; KCC2, potassium-chloride co transporter 2; MC, mast cells; ATP, adenosine triphosphate; A1Rs, A1 receptors; Aδ, A delta; GABA, gamma-aminobutyric acid; NA, noradrenaline; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B.