Figure 2. BI stabilizes Broccoli.
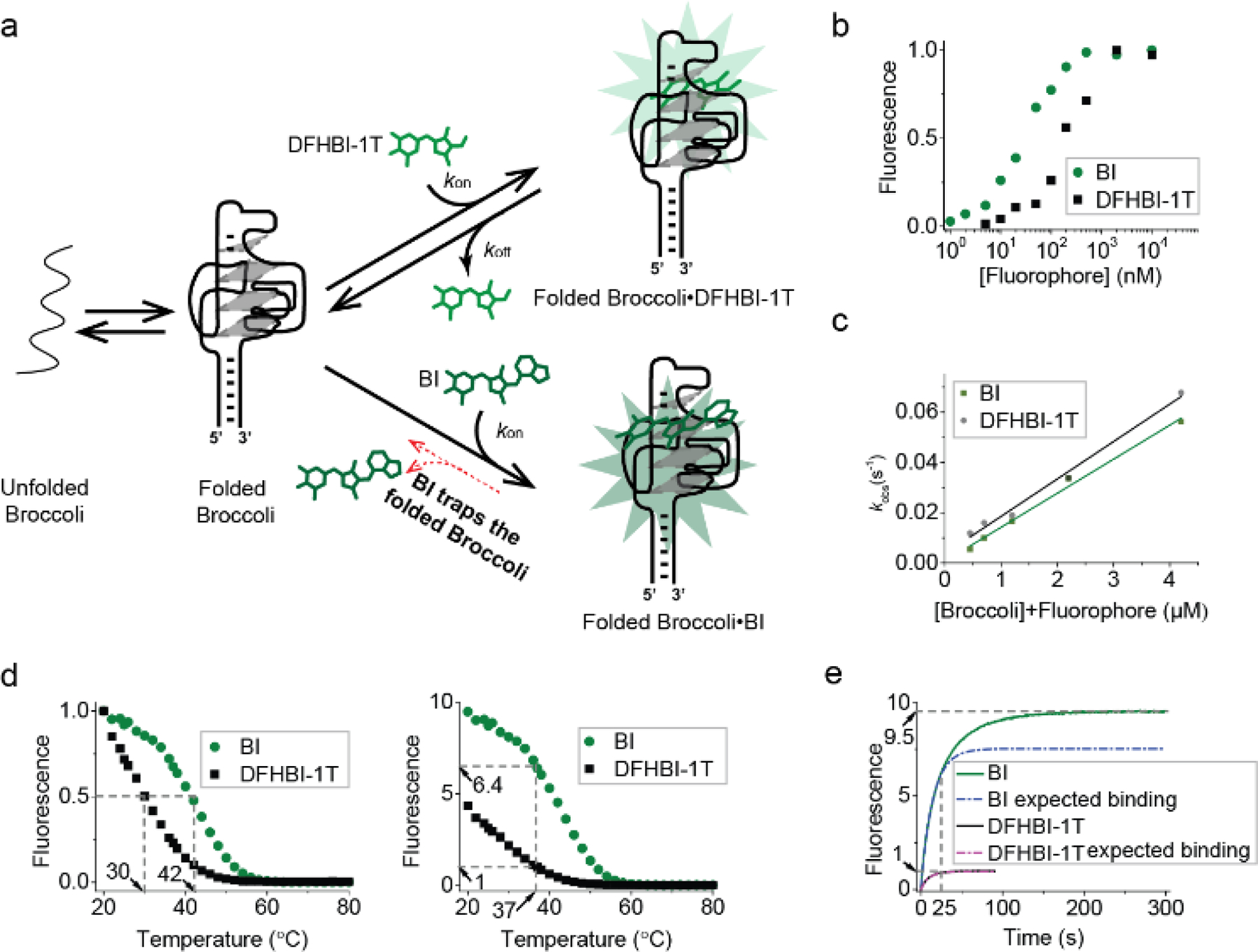
(a) Model for how BI could enhance overall cellular fluorescence by stabilizing Broccoli in folded form. (b) Broccoli (50 nM) was titrated with either DFHBI-1T or BI at 25°C and then fitted using the Hill equation as described previously.[1a] BI (KD= 51 nM), DFHBI-1T (KD= 305 nM). (c) kon and koff were calculated from the linear fit (solid line) of kobs vs. concentration at 25°C. The slope provides kon and the intercept gives koff. kon=14,900 M-1 s-1, koff=0.0036 s-1 for DFHBI-1T and kon=13,600 M-1 s-1, koff=0.0006 s-1. (d) The fluorescence of a solution containing 25 μM RNA and 500 nM BI or DFHBI-1T was measured in 1 mM MgCl2 buffer while gradually increasing the temperature. In the left image, the fluorescence is normalized so that both Broccoli-BI and Broccoli-DFHBI-1T start at 1.0. Broccoli-BI exhibited a ~12°C shift in the Tm relative to Broccoli-DFHBI-1T (left). On the right, the fluorescence is normalized by the fluorescence level of Broccoli-DFHBI-1T at 37°C. (e) Fluorescence intensity upon mixing Broccoli and BI or DFHBI-1T (final concentration: 25 μM Broccoli, 0.5 μM fluorophore) at 37°C (0.2 mM MgCl2 buffer). Upon adding BI to Broccoli, an initial (0–25 sec) rapid increase (k1=0.06771 s-1) in fluorescence was observed (expected binding based on a pseudo-first order binding reaction indicated as a dotted line, the actual observed fluorescence increase is shown in solid color). BI exhibited a continued increase in fluorescence consistent with a second mode of binding (k2=0.02756 s-1) with a plateau at ~200 sec. The rate constants (k1 and k2) were modeled by a single exponential fit in Origin software.
