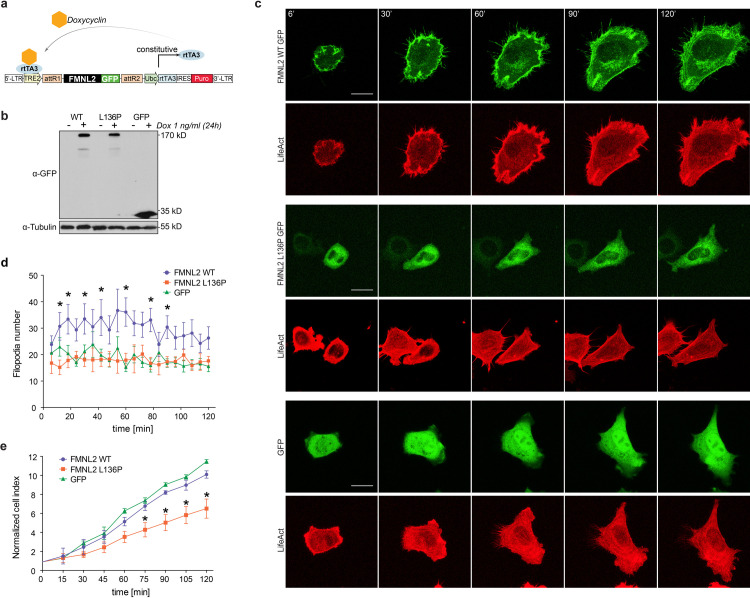
Fig 3. L136P disturbs cell spreading and filopodia formation.
a) The pInducer20 Puro expression vector [21] allowed for a doxycycline-induced expression of the FMNL2-GFP variants and the appropriate GFP-control. Doxycycline acts as a cofactor for constitutively expressed reverse tetracycline-controlled transactivator (rtTA3) at the tetracycline response element (TRE2). Following lentiviral transduction, puromycin selection was made possible by the expression of a puromycin resistance gene (Puro). (modified from [21]). b) The Immunoblot shows the inducible expression of the indicated gene variants in stable NIH/3T3 cell lines without (-) and with (+) Doxycyclin treatment as indicated. c) Live cell imaging of NIH/3T3 cells was performed to visualize the dynamics of the spreading process. The cells stably expressed the indicated inducible GFP-tagged variants and LifeAct for visualization of actin-filaments. Stills represent the indicated time points over the course of a 120 minute imaging time. Scale bar = 20 μm d) Image-based quantification of filopodia formation during the 120 min spreading process in NIH/3T3 cells (as shown in b). n = 10. (p-values for individual time points, L136P vs. WT: 12’ p = 0,017; 18’ p = 0,022; 30’ p = 0,023; 42’ p = 0,044; 60’ p = 0,007; 78’ p = 0,004; 90’ p = 0,012). e) Impedance analysis was performed to assess the dynamic aspects of cell spreading. 5.000 NIH/3T3 cells were seeded in triplicates on 96-well E-plates and impedance measurements were taken every 15 minutes. Normalized cell index indicates the relative increase of impedance that correlates with the mean area of the plated cells (p-values of individual time points, L136P vs. WT: 75’ p = 0,048; 90’ p = 0,022; 105’ p = 0,039; 120’ p = 0,029).