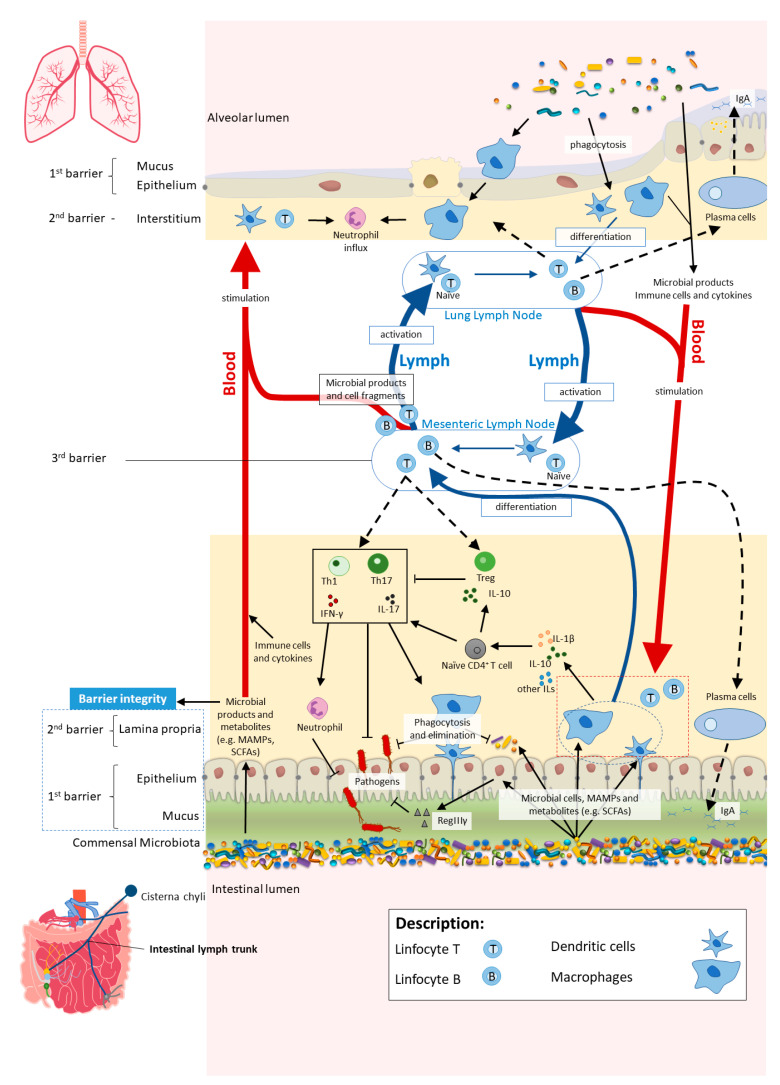
Figure 2.
In homeostasis, microbe-associated molecular patterns (MAMPs) from the gut microbiota are recognised by pattern recognition receptors (e.g., Toll-like receptors (TLRs)) and induce antigen presenting cells (APCs), such as macrophages and dendritic cells (DC), to produce interleukins (e.g., IL- 1β and IL-10) to regulate immune responses by different subsets of T cells, neutrophils and macrophages, among others. Activated APCs induce differentiation of naïve CD4+ T cells into CD4+ regulatory T cells (Treg) (which are crucial for both maintaining tolerance to commensal microbiota and regulating other immune cells), and other effector T cells such as Th1 or Th17 (expressing cytokines, e.g., IL-17 and interferon gamma (IFNγ)) with a central role in host defence against invading pathogens, while controlling the expansion of commensals. Microbial cells or their products in the lamina propria are either phagocytosed and eliminated or transferred to mesenteric lymph nodes (MLN) by APCs, where they induce differentiation of the T and B cells. Activated B and T cells move back (black dashed arrows) to the intestinal mucosa to directly act on their target or to continue to trigger other immune cells. The majority of activated B cells differentiate into immunoglobulin A (IgA)-producing plasma cells. Bacterial metabolites, such as short-chain fatty acids (SCFAs), and expression of antimicrobial peptides (e.g., RegIIIγ) by epithelial cells (induced by TLR activation by MAMPs) reinforce the intestinal barrier integrity. Proposed pathways of the gut–lung axis that would explain the impact of the gut microbiota on the lung immunity include the migration of: (1) activated T and B cells from the MLN to distal sites such as the lung epithelium and lung lymph nodes, through lymph and blood; (2) microbial products and metabolites or surviving bacteria from the intestinal mucosa to the lung, through systemic propagation by lymph and blood circulations. Although not yet well established in the literature, the other way around has been proposed as well (from lung to gut), with the lung microbiota exerting effects in the intestinal mucosa. Scheme based on Bingula et al. [52].