Optimization of the reaction conditionsa.
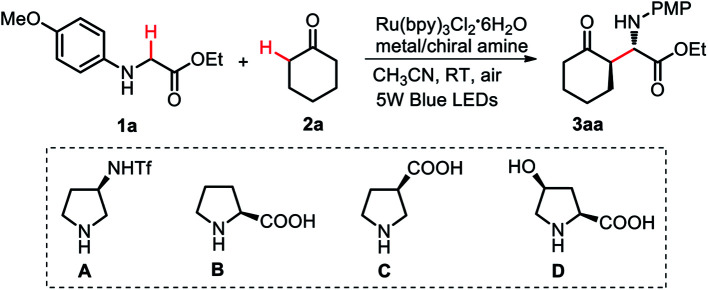
| ||||
|---|---|---|---|---|
| Entry | Amine/metal | Yieldb (%) | d.r.c (anti/syn) | eed (%) |
| 1 | A/Cu(OAc)2·H2O | 76 | 97/3 | 96 |
| 2 | B/Cu(OAc)2·H2O | n.d. | — | — |
| 3 | C/Cu(OAc)2·H2O | 57 | 98/2 | 88 |
| 4 | D/Cu(OAc)2·H2O | n.d. | — | — |
| 5 | A/Zn(OAc)2 | n.d. | — | — |
| 6 | A/Cu(OTf)2 | 63 | 98/2 | 86 |
| 7 | A/CuSO4 | 68 | 97/3 | 92 |
| 8 | A/CuI | 63 | 97/3 | 92 |
| 9e | A/Cu(OAc)2·H2O | 80 | 97/3 | 96 |
| 10e,f,g | A/Cu(OAc)2·H2O | 81 | 98/2 | 97 |
| 11e,f,h | A/Cu(OAc)2·H2O | 47 | 95/5 | 94 |
| 12e,f,g,i | A/Cu(OAc)2·H2O | 82 | 98/2 | 97 |
| 13j | A/Cu(OAc)2·H2O | n.r. | — | — |
| 14 | A/- | n.d. | — | — |
| 15e,f,k | A/Cu(OAc)2·H2O | n.r. | — | — |
| 16e,f,l | A/Cu(OAc)2·H2O | n.r. | — | — |
Reaction conditions: 1a (0.1 mmol), Ru(bpy)3Cl2·6H2O (5 mol%), Lewis acid (10 mol%), CH3CN (2.0 mL), 5 W blue LEDs light irradiation under air at room temperature for 2 h, then 2a (0.5 mmol) and chiral amine catalyst (20 mol%) were added.
Combined yield of isolated 3aa (syn and anti).
Determined by HPLC on a chiral stationary phase.
Determined by HPLC on a chiral stationary phase.
2 mol% of Ru(bpy)3Cl2·6H2O was used.
5 mol% of Cu(OAc)2·H2O was used.
1.5 mL CH3CN was used.
10 mol% of A was used.
Reaction was carried out under O2 atmosphere.
Reaction was performed without Ru(bpy)3Cl2·6H2O.
Reaction was carried out in the dark.
Reaction was carried out under argon. n.d. = not determined. n.r. = no reaction.
