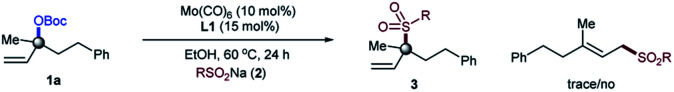
Sodium sulfinate substrate scopea,b,c.
| |||
|---|---|---|---|
| Entry | 2 | 3 b | Yieldc (%) |
| 1 | 2a (R = Ph) | 3aa | 92 |
| 2 | 2b (R = 4-MeC6H4) | 3ab | 93 |
| 3 | 2c (R = 4-MeOC6H4) | 3ac | 90 |
| 4 | 2d (R = 4-ClC6H4) | 3ad | 87 |
| 5 | 2e (R = 4-FC6H4) | 3ae | 85 |
| 6 | 2f (R = 4-NO2C6H4) | 3af | 75 |
| 7 | 2g (R = 4-CNC6H4) | 3ag | 72 |
| 8 | 2h (R = 2-FC6H4) | 3ah | 88 |
| 9 | 2i (R = 2-ClC6H4) | 3ai | 87 |
| 10 | 2j (R = 2-OCF3C6H4) | 3aj | 72 |
| 11 | 2k (R = 3-BrC6H4) | 3ak | 82 |
| 12 | 2l (R = 3-CNC6H4) | 3al | 78 |
| 13 | 2m (R = 2,4-MeOC6H3) | 3am | 94 |
| 14 | 2n (R = 3,5-CF3C6H3) | 3an | 95 |
| 15 | 2o (R = 2-MeO, 5-BrC6H3) | 3ao | 84 |
| 16 | 2p (R = 3,4-ClC6H3) | 3ap | 87 |
| 17 | 2q (R = 2-naphthyl) | 3aq | 82 |
| 18 | 2r (R = 1-quinoline) | 3ar | 78 |
| 19 | 2s (R = 2,3-dihydrobenzofuran) | 3as | 92 |
| 20 | 2t (R = 3-pyridine) | 3at | 82 |
| 21 | 2u (R = 2-thiophene) | 3au | 86 |
| 22 | 2v (R = Me) | 3av | 72 |
| 23 | 2w (R = Et) | 3aw | 78 |
| 24 | 2x (R = iPr) | 3ax | 82 |
| 25 | 2y (R = cyclopropyl) | 3ay | 78 |
| 26 | 2z (R = CH3OCOCH2CH2) | 3az | 72 |
Reaction conditions: Mo(CO)6 (10 mol%), L1 (15 mol%), 1a (0.2 mmol), RSO2Na 2 (0.3 mmol), EtOH (1.0 mL, 0.2 M), 60 °C, 24 hours.
Determined by 1H-NMR of the crude reaction mixture.
Isolated yields.
