Figure 1.
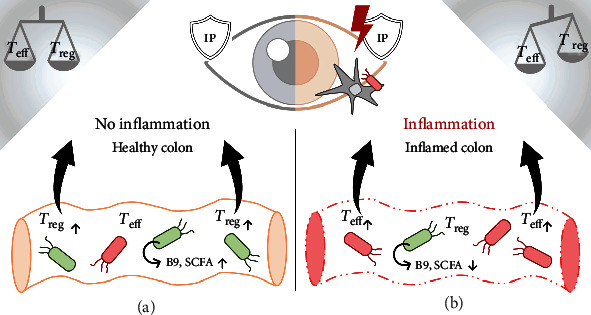
Bioavailable folate (B9) produced by certain phyla of the human microbiome (Proteobacteria, Firmicutes, Actinobacteria, and Verrucomicrobia [84]; green microbes) and short-chain fatty acids (SCFA) locally stabilize T regulatory cells (Treg) in the colon, thereby increasing their abundance (a). T cells traffic from the colon to distant sites [85, 86] where they accumulate and exert their respective functional properties. The inflamed colon ((b); characterized by structural damage—“leaky gut”) is frequently accompanied by dysbiosis (red microbes). This qualitative and quantitative shift in bacterial colonization is associated with decreased microbial folate and SCFA production and a consequential relative increase in autoreactive immunogenic T effector cells (Teff) [87]. Teff have been shown to traffic from the colon to target sites of autoimmunity [85, 86] (e.g., intraocular tissue in the case of autoimmune uveitis), skewing the ratio of immunogenic (Teff) to regulatory cells (Treg) at target sites [88–90], ultimately breaching ocular immune privilege (IP) through unknown mechanisms, and thereby triggering autoimmune disease. Due to impaired intestinal barrier integrity in dysbiosis, pathogenic bacterial/viral/fungal/environmental antigens have facilitated access to the circulation, possibly triggering inflammation through adjuvant effects at affected sites (following antigen presentation; APC) (b). In the case of uveitis, we propose that when a sufficiently high Teff precursor frequency is generated [91], activated T cells access retinal tissue where they adopt a pathogenic phenotype upon further activation by retinal self-antigen and/or microbial antigen [92].
