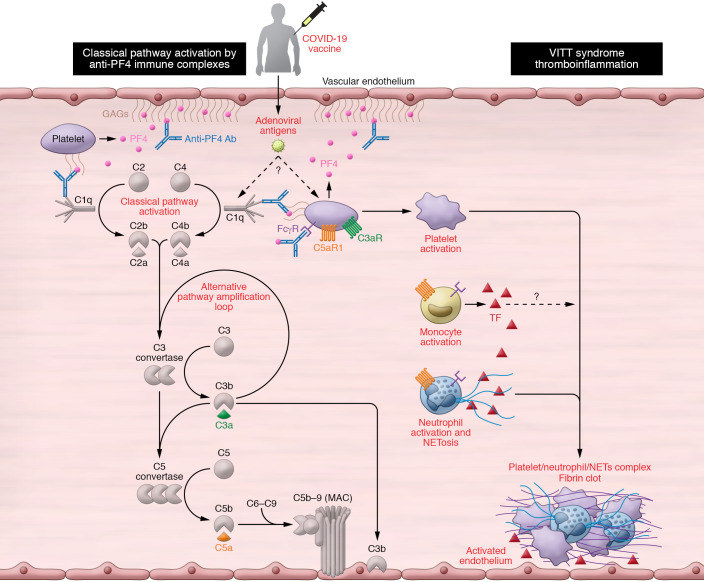
Figure 1. A schematic diagram of the plausible mechanisms by which complement may contribute to the VITT-associated prothrombotic response.
Vaccine-induced immune thrombotic thrombocytopenia (VITT) has been described in individuals presenting with high titers of platelet-activating anti–platelet factor 4 (PF4) autoantibodies. These antibodies recognize large multiantigenic complexes comprising PF4 and polyanionic structures similar to heparin, and resemble autoimmune heparin-induced thrombocytopenia (HIT). Complement activation can be triggered in multiple ways in patients presenting with VITT and anti-PF4 antibodies. First, the formation of complexes between PF4 and certain vaccine constituents (e.g., adenoviral capsid proteins or DNA) (23) may serve as a scaffold for C3 activation, similarly to what has been observed with PF4-heparin ultra-large complexes. In addition, complement activation can be triggered by the binding of C1q to the anti-PF4 immune complexes that are deposited on the endothelium, monocyte, or platelet surface via binding to polyanionic structures such as glycosaminoglycans (GAGs). Classical pathway activation leads to C3 cleavage, amplification of complement responses via the alternative pathway, downstream generation of proinflammatory C3a and C5a anaphylatoxins, and the formation of the C5b-9 complex. Complement activation fragments mediate a broad range of thromboinflammatory reactions by interacting with complement receptors on platelets, monocytes, and neutrophils. These interactions can induce or enhance FcR-mediated platelet activation, neutrophil-platelet aggregation, and release of TF-loaded neutrophil extracellular traps (NETs) from activated neutrophils. All these processes are fueled by complement activation and can collectively contribute to a prothrombotic environment that may lead to VITT. Moreover, C3-opsonized immune complexes can enhance FcγR-dependent effector responses on platelets further promoting thrombotic responses. Abbreviations: CP, classical pathway; AP, alternative pathway; TF, tissue factor.