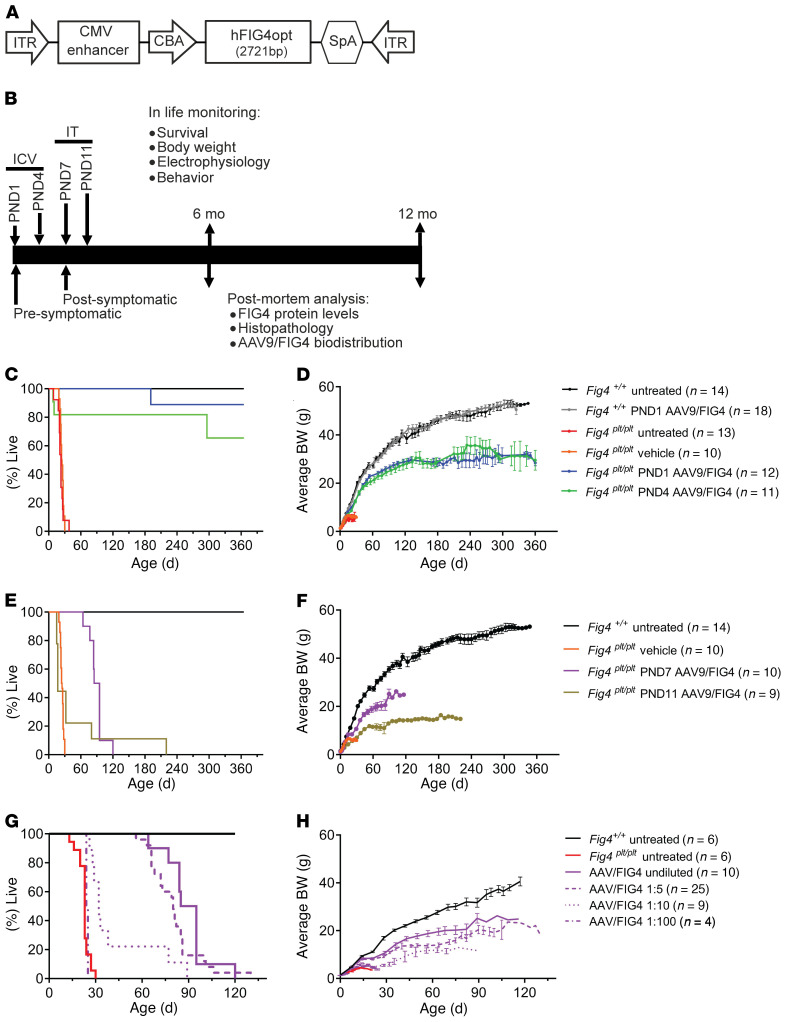
Figure 1. Neonatal delivery of AAV9-FIG4 vector improves survival of Fig4plt/plt mice.
(A) Representation of the AAV9-FIG4 vector, with a full-length, codon-optimized human FIG4 cDNA (hFIG4opt) under control of the CBA promoter, including a CMV-derived enhancer and a synthetic poly(A) tail (SpA). This expression cassette was flanked by an ITR sequence for packing into AAV9. (B) Outline of preclinical study design. Fig4plt/plt mice were treated by i.c.v. injection at P1 or P4 with AAV9-FIG4, representing the presymptomatic intervention and proof-of-concept group for efficacy testing. Fig4 WT littermates were dosed i.c.v. at P1, representing the safety group for AAV9-FIG4 toxicity evaluation. Both Fig4plt/plt and WT mice treated with vehicle or untreated were included as controls. For postsymptomatic treatments, Fig4plt/plt mice were treated at P7 or P11 via i.t. injection. A minimum of n = 5 mice per sex per genotype were enrolled in each group. All mice were monitored daily for body weight and survival. Electrophysiology and behavioral assessments were conducted at various time points after AAV9-FIG4 treatment. Some of the i.c.v. injected group were analyzed at 6 to 8 months after treatment and the rest at the 12- to 13-month end point. (C) Survival and (D) average body weights (BW) for mice injected with AAV9-FIG4 via i.c.v. (5.4 × 1011 vg) at P1 and P4. For postsymptomatic intervention, Fig4plt/plt mice were treated by i.t. injection (AAV9-FIG4, 1.35 × 1012 vg) at P7 and P11. (E) Survival and (F) average body weights are shown. Dose-response analysis was conducted by injecting Fig4plt/plt mice i.t. with AAV9-FIG4 diluted 1:5 (2.7 × 1011 vg), 1:10 (1.35 × 1011 vg), and 1:100 (1.35 × 1010 vg). (G) Survival and (H) average body weights are shown. The log-rank (Mantel-Cox) test was applied for survival curve comparisons. Growth curves show average body weight ± SEM.