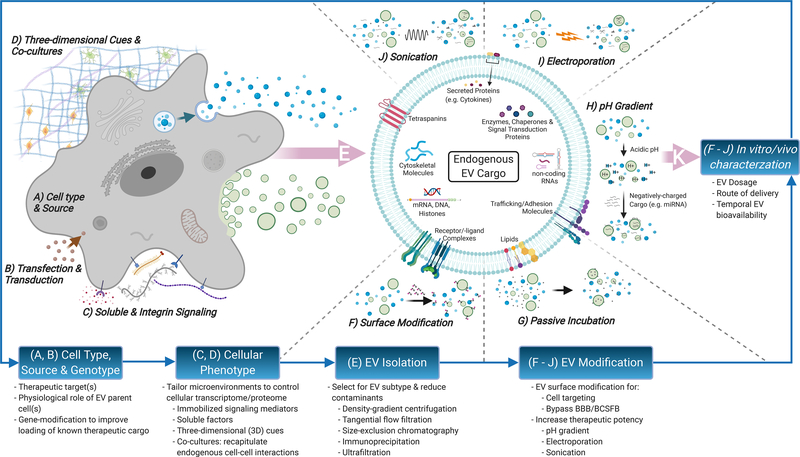
Figure 3: Strategies for enhancing CNS-specific regenerative potential of EV-based SCI therapeutics.
Engineering approaches to probe for populations of therapeutic EVs begin with selection of therapeutic target(s) for SCI (e.g. neurogenesis, axonal sprouting, remyelination, etc.) matched with appropriate EV producer cell types hypothesized to play direct/indirect physiological roles (A). EV producer cells may be genetically modified to further enhance EV loading of known biological mediators (B). Control over cellular phenotype through immobilized signaling mediators (e.g. extracellular proteins) or soluble factors (e.g. cytokines, hormones, etc.) can modify cargo and bioactivity of secreted EV populations (C). Additionally, biophysical cues such as three-dimensional culture and incorporation of specific cell-cell interactions using co-cultures (e.g. neuronal/oligodendroglial co-cultures to target remyelination) can further tune loading of EV-associated cargo (D). EVs are highly heterogeneous in size, membrane composition, cargo, and bioactivity. Many of these parameters are closely linked to the EVs’ biogenesis pathways, which include: i) release of intraluminal vesicles within multivesicular bodies (MVBs) upon fusion with the plasma membrane, ii) direct budding of the plasma membrane and, iii) formation of apoptotic bodies. A significant hurdle in current EV isolation techniques is the inability to robustly differentiate between these EV subtypes. Thus, isolated EV populations will inevitably contain particles originating from multiple biogenesis pathways (E). Overall functional cargo makeup on EV surface (F) and/or EV interior (G-J) can also be modified to varying degrees after EV biogenesis and isolation. Lastly, in vitro/vivo characterization of biomanufactured EVs can inform further modifications to optimize for therapeutic efficacy and potency (K). Principal considerations for in vitro characterization include cell-based assays that probe for relevant EV bioactivity (e.g. neuroprotection, neuroinflammation). Considerations for in vivo characterization include selection of SCI injury model, EV dosage, route of delivery to the CNS and temporal bioavailability of delivered EVs over an appropriate therapeutic time window.