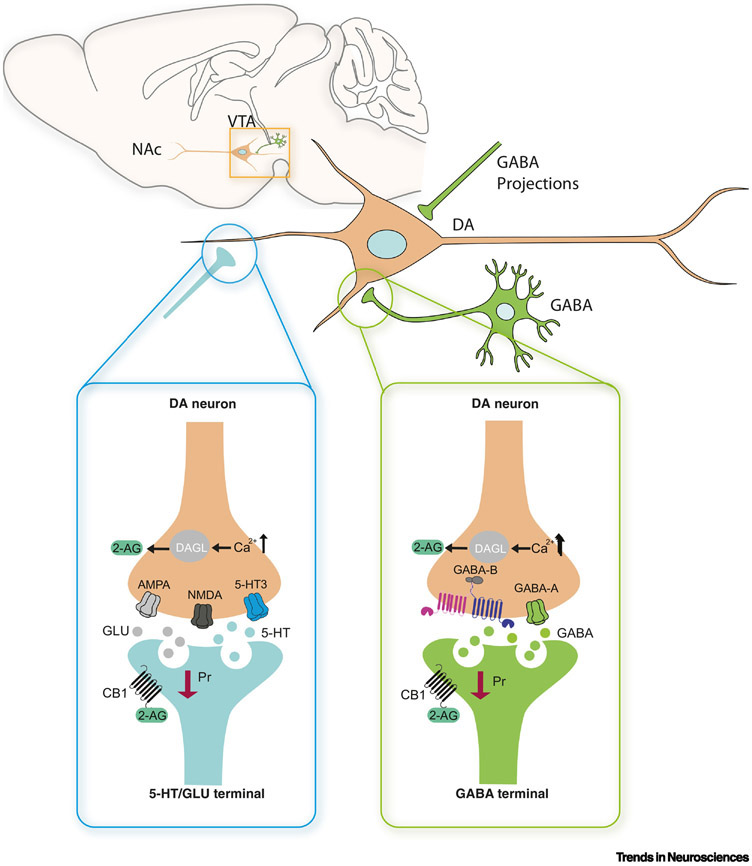
Figure 2. Modulation of VTA neurons by DRN and GABAergic inputs, as well as local interneurons.
The schematic illustrates a VTA dopamine neuron (orange) projecting to the NAc, and receiving synaptic inputs from a 5-HT/GLU DRN neuron (cyan) and a local GABAergic neuron (green). Left inset: DRN to VTA projections modulate dopamine release and are regulated by CB1 receptors. Axonal terminals of DRN 5-HT neurons within the VTA can co-release 5-HT and glutamate (GLU); this signal is transduced by 5-HT receptors (specifically 5-HT3) and AMPA and NMDA receptors on dopaminergic neurons (DA neurons) [51]. Excitation of VTA DA neurons by DRN terminals results in raised intra-cellular calcium (Ca2+) and leads to the production of ECs (eg. 2-AG). CB1 receptors on these 5-HT/glutamate terminals within the VTA are activated by 2-AG. CB1 activation in turn decreases the probablity of neurotransmitter/neuromodulator release (Pr), dampening the drive onto DA neurons.
Right inset: GABAergic projections to VTA dopamine neurons are regulated by CB1 receptors, resulting in disinhibition of dopamine release. DA neurons within the VTA are normally inhibited by GABA, but regulation via the EC system provides a disinhibitory mechanism. That is, when DA neurons fire in high frequency bursts, intracellular Ca2+ levels increase. As a result, 2-AG is synthesized and released into the extrasynaptic space. 2-AG retrogradely activates CB1 receptors on GABAergic terminals and GABA release is suppressed. The resulting disinhibition of the dopamine neuron increases dopamine activity and consequent terminal release.