Table 2.
Scope of the Biginelli condensation for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones catalyzed by MCM-41-APS-PMDA-NHSO3H (1)a.
| Entry | Aldehyde 3 | Product 5 | Time (min) | Yield (%)b | mp °C (Obs.) | mp °C (Lit.) |
|---|---|---|---|---|---|---|
| 1 | 4-ClC6H4– |
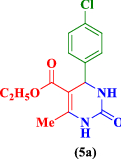
|
35 | 95 | 210–211 | 210–21172 |
| 2 | C6H5– |
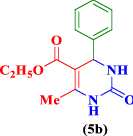
|
55 | 87 | 235–236 | 234–23673 |
| 3 | 4-NO2C6H4– |
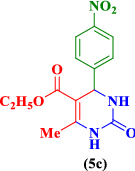
|
50 | 80 | 204 | 224–22774 |
| 4 | 3-NO2C6H4– |
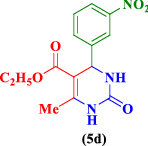
|
60 | 82 | 293–295 | 20475 |
| 5 | 4-CH3OC6H4– |
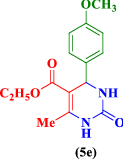
|
55 | 89 | 201–203 | 202–20476 |
| 6 | 2-ClC6H4– |

|
60 | 90 | 211–213 | 211–21375 |
| 7 | 4-OHC6H4– |
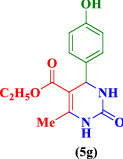
|
55 | 82 | 234–236 | 233–23577 |
| 8 | 2-C4H3S– |
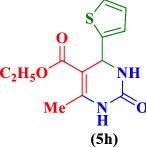
|
45 | 84 | 212–214 | 210–21278 |
| 9 | 4-Me2NC6H4– |
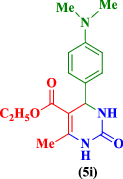
|
45 | 92 | 213–215 | 213–21579 |
| 10 | 4-FC6H4– |
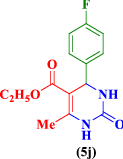
|
60 | 85 | 180 | 18080 |
| 11 | 4-OH-3-MeO-C6H3– |
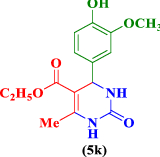
|
40 | 84 | 188–190 | 188.581 |
aReaction conditions: ethyl acetoacetate (2, 1 mmol), aldehydes (3a–k, 1 mmol), urea (4, 1.2 mmol), MCM-41-APS-PMDA-NHSO3H (1, 15 mg) under solvent-free conditions at 80 °C.
bIsolated yields were reported.
