Abstract
Pollutants found in the water and air environment represent an ever-growing threat to human health. Contact with some air-, water- and foodborne pathogens (e.g. norovirus) results in gastrointestinal diseases and outbreaks. For future risk mitigation, we aimed to measure people’s awareness of waterborne and foodborne norovirus relative to other environment-associated pollutants (e.g. pesticides, bioaerosols, antibiotic resistant bacteria) and well-known risks (e.g. diabetes, dementia, terrorist attack). We used an online survey, which included a best–worst scaling component to elicit personal levels of control and fear prompted by norovirus relative to 15 other risks. There was a negative correlation between levels of fear vs. control for all 16 measured risks. Perceived infection control levels were higher amongst women compared to men and correlated with age and the level of qualification in both groups. Participants who had sought advice regarding the symptoms caused by norovirus appeared to have more control over the risks. Norovirus is associated with high levels of fear, however, the levels of control over it is low compared to other foodborne illnesses, e.g. Salmonella. Addressing this deficit in the public’s understanding of how to control exposure to the pathogen in an important health need.
Subject terms: Policy and public health in microbiology, Virology, Environmental social sciences, Risk factors
Introduction
Increased agricultural and industrial activities alongside progressive urbanization have caused the emergence and spread of a wide range of pollutants in the environment, leading to global health threats. For example, air pollutants (including bioaerosols) and pesticides are leading to an increasing number of skin and respiratory syndromes1–4. The overuse of antibiotics in healthcare and agriculture has led to a high prevalence of antibiotic resistant bacteria (‘superbugs’) resulting in an increasing number of untreatable bacterial infections5.
The number of infections and outbreaks associated with gastrointestinal symptoms are also on the rise6–9. Among these pathogens, norovirus (often known as ‘winter vomiting bug’) is the most common cause of non-bacterial gastroenteritis globally10,11. Norovirus is highly infectious, spreads rapidly and hence is responsible for outbreaks leading to the temporary closure of public places (e.g. schools, restaurants and hospitals). The symptoms, including diarrhea, vomiting, nausea, fever and abdominal pain, are usually mild and resolve in 2–5 days, however, in some cases, the infection can be life threatening10. The estimated annual number of cases is 685 million with approximately 200,000 deaths worldwide10,12 with a total of US$4.2 billion in direct health system costs and US$60.3 billion in societal costs13.
The main route of transmission is direct (person-to-person) contact, however, the number of cases associated with consumption of contaminated food and water is increasing14,15. Norovirus can be found at high concentrations in the stools of infected individuals even weeks after the symptoms are resolved16. As the virus is extremely resistant to traditional wastewater treatment procedures17–19, significant loads are discharged into the environment contaminating recreational and drinking water bodies and irrigation systems20–22. Furthermore, norovirus is transported in environmental waters over long distances, accumulating in sediment23 and taken up by filter feeder aquatic animals, such as shellfish, harvested for human consumption24,25. As little is known about the transport patterns and behavior of norovirus, the contamination and health risks associated with water and shellfish are hard to predict26.
As the virus is highly contagious and no vaccination is available, prophylactic measures are critical to the control of disease spread27,28. For instance, in some developed countries, information on the risks associated with norovirus and other foodborne agents are disseminated in public places (e.g. healthcare units, pharmacies, restaurant menus) and on governmental websites. Research has been done to understand the persistence and associated risks of norovirus and other pollutants in the environment26,29–33. Several studies have attempted to evaluate the perception of food safety and microbial contamination in general34–37. However, little is known about people’s awareness and perception of norovirus and its transmission38–40 despite the importance of such knowledge in shaping the behaviors which may reduce the spread of the disease.
This study investigated the public’s awareness and perceptions of norovirus and other environment-associated health risks. This was undertaken via analysis of data from a UK national survey. Within this survey, a choice-based approach (Best Worst Scaling; BWS)35 was used to determine the levels of fear and control people associated with norovirus in comparison to a set of other risks. Specifically, we investigated:
-
I.
the level of public awareness of norovirus
-
II.
the level of fear associated with norovirus compared to other hazards (both environment-related and more general) with established perception
-
III.
the level of control people believed they had over norovirus compared to other hazards (both environment-related and more general) with established perception
-
IV.
how these perceptions of control and fear varied over socio-demographic characteristics and the implications of these variations for efforts to increase awareness of norovirus and reduce the disease burden.
Results
The survey questions and results from respondents are available at the Environmental Information Data Centre (EIDC, www.eidc.ceh.uk). https://doi.org/10.5285/0869d961-99ca-4946-9192-f35afccdda38.
Respondents’ characteristics and experience with norovirus sources
The online survey was completed by 1006 adults between the 15th and 20th February 2018. In order to assess the awareness of people most vulnerable to water- and foodborne illnesses, individuals who had consumed bivalve shellfish and had been in contact with environmental waters within the previous year were asked to participate. After the removal of incomplete responses, those who had completed the survey so quickly as to suggest inadequate consideration of their responses (n = 194), the sample comprised 806 responses (80% of total responses). Of those 806 responders, 47% were male, 96% white, 7% were 18–24 years old, 30% were aged 24–44 years old, 38% were 45–64 years old and 25% were over 65 (Table S1). Of the respondents, 72% were parents or legal guardians with 62% having one child and 19% having two children. 47% of the children were older than 7 years (25% of the children were 7–12 and 22% were 13–18 years old).
The respondents consumed shellfish cooked in a restaurant or food outlet (89%, n = 718), at home (79%, n = 636), as ready meals (68%, n = 548) or as pickles (54%, n = 437) in the past year. Approximately one half of the sample consumed shellfish raw in restaurants (48%, n = 387) and a third consumed them raw at home (32%, n = 255). Of all respondents, 16% (n = 125) believed that they had contracted gastroenteritis (defined as “upset stomach”, see “Methods” section for details) due to shellfish consumed in a restaurant in the UK or abroad (9%, n = 70 and 5%, n = 37, respectively), takeaway in the UK or abroad (5%, n = 38 and 3%, n = 22, respectively) or as a home-cooked meal in the UK or abroad (4%, n = 34 and 0.6%, n = 5, respectively). Among the 125 respondents who believed their illness associated with consuming shellfish, most respondents started to make sure the shellfish they were eating were well cooked (75%, n = 94), told others about the experience (61%, n = 76), had a break from eating shellfish (61%, n = 76) or raw shellfish (43%, n = 54), permanently stopped eating raw shellfish of any kind (40%, n = 50) or the type that made them sick (40%, n = 50), or permanently stopped eating shellfish at home (27%, n = 34) or in a restaurant (28%, n = 35).
The majority of the respondents (87%, n = 701) had contact with UK recreational waters 4–5 times a year and 29% (n = 234) of the respondents had experienced gastroenteritis-related illness after the activity. Due to the illness, 60% (n = 140) of those people started to use hand sanitizers after recreational activity, 52% (n = 121) told others about the experience, 51% (n = 119) took a break from using recreational waters, 38% (n = 90) changed the places they visited and 24% (n = 57) changed or stopped their recreational activities. Of all 806 respondents, 22% (n = 178) had contracted gastroenteritis after nursing someone with gastroenteritis in the UK and 14% (n = 113) became ill after taking care of a sick person abroad.
Respondents’ awareness of norovirus
Over the previous year, 40% (n = 324) of the respondents had looked for advice or guidance regarding gastroenteritis (Table 1). Of those who visited one or more of the governmental websites (National Health Services choices website, Public Health England and Wales, Health Protection Scotland, Food Standards Agency, Food Standard Scotland), 69% (n = 97) found the information useful and all would use these resources in the future. Of those who did not use governmental websites as resources, 74% (n = 136) were not aware of the resources and indicated that they would use these resources in the future. Of those respondents who did not look for advice on governmental websites (Table 1), 27% (n = 216) would use governmental websites. Of those who would not use governmental websites, 77% (n = 454) were not aware of the resources, 12% (n = 71) believed those resources would not be useful and 11% (n = 65) were not interested or would rather ask a health care professional in person.
Table 1.
Summary on the use of information sources on gastroenteritis.
| Sought information on gastroenteritis (n = 324), n(%) | Would consider seeking information on gastroenteritis (n = 482), n(%) | |
|---|---|---|
| Accident and Emergency Department | 43 (13%) | 42 (9%) |
| General practitioner/family doctor | 121 (37%) | 238 (49%) |
| Medical center | 47 (15%) | 127 (26%) |
| NHS 111 | 52 (16%) | 215 (45%) |
| NHS Choices website | 105 (32%) | 188 (39%) |
| PHE/PHW/HPS website | 45 (14%) | 57 (12%) |
| FSA/FSS website | 24 (7%) | 33 (7%) |
| Newspapers | 15 (5%) | 7 (1%) |
| Online newspapers | 22 (7%) | 16 (3%) |
| Friends, family social media | 77 (24%) | 117 (24%) |
| Internet | 112 (35%) | 184 (38%) |
| Pharmacy | 7 (2%) | 43 (9%) |
| Other | 4 (1%) | 0 (0%) |
NHS: National Health Service; PHE: Public Health England; PHW: Public Health Wales; HPS: Health Protection Scotland; FSA: Food Standards Agency; FSS: Food Standards Scotland.
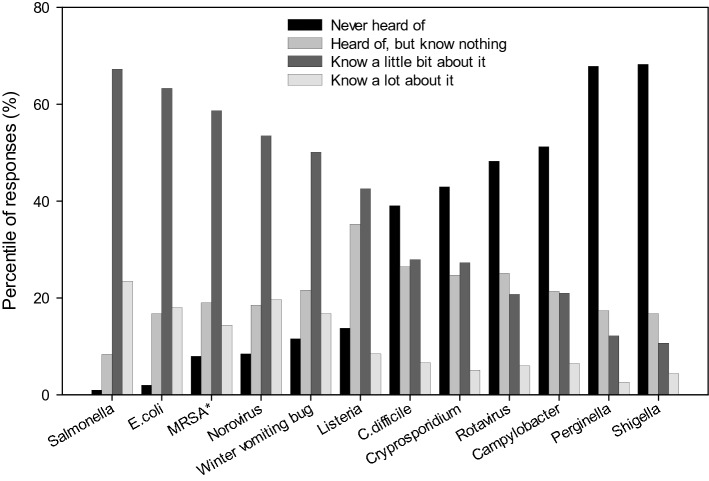
The respondents were also asked about their knowledge of pathogens associated with gastroenteritis, including a fictive pathogen (Perginella) as a control. Salmonella was the pathogen which most people indicated they knew about followed by E. coli (Fig. 1). Norovirus was ranked 3rd (with MRSA) with 73% claiming to know a little or a lot about it. The equivalent figure for ‘winter vomiting bug’ (synonym for norovirus) was 67%. Listeria was ranked 6th and Campylobacter ranked 9th (21% said they knew a little about it, 6% claimed to know a lot) along with rotavirus, Shigella and the fictive pathogen, Perginella was reported as the least-known pathogen, with 3% of respondents claiming to know a lot about it.
Figure 1.
Respondents’ knowledge on pathogens causing gastroenteritis. *Methicillin-resistant Staphylococcus aureus.
Respondents’ perceptions of fear
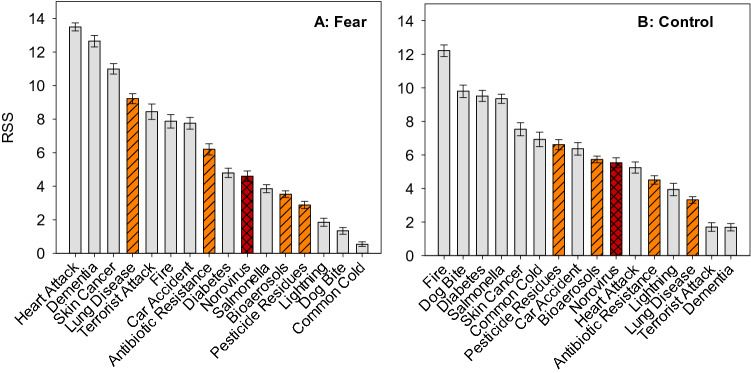
The respondents’ ratio-scaled fear and control scores were determined based on the BWS experiment. The BWS part included 16 risks, such as norovirus, other air, food and waterborne illnesses and other hazards (Table 2). Ratio-scaled fear scores from estimation of the mixed logit model are reported in Table 3 and displayed in Fig. 2A. The most feared risk was heart attack, followed by getting dementia and becoming ill with skin cancer. The most feared environmental risk, ranked 4th, was getting lung disease from air pollution. Norovirus was ranked 10th in terms of fear, with a level of fear equal to that from diabetes. The fear associated with norovirus was 18% greater than associated with Salmonella, 30% greater than that from bioaerosols, and 60% greater than the fear of illness from pesticide residues. We have found no significant differences in the fear levels of people with different age, gender, education, employment status, family background, income or ethnicity.
Table 2.
Risks used in the best worst scaling (BWS).
| No | Variable name | Risk as defined in the BWS tasks |
|---|---|---|
| 1 | Terrorist attack | Being a victim of a terrorist attack |
| 2 | Dementia | Getting dementia in your lifetime |
| 3 | Car accident | Being injured in a car accident |
| 4 | Lung disease | Getting lung disease as a result of air pollution |
| 5 | Antibiotic resistance | Getting ill from bugs that are not killed by antibiotics |
| 6 | Fire | Fire at home |
| 7 | Diabetes | Getting diabetes in your lifetime |
| 8 | Heart attack | Suffering a heart attack in your lifetime |
| 9 | Pesticide residues | Becoming ill from eating substances that control pests or weeds that would remain on or in food |
| 10 | Skin cancer | Getting skin cancer in your lifetime |
| 11 | Common cold | Getting the common cold |
| 12 | Dog bite | Being bitten by a dog |
| 13 | Bioaerosols | Having breathing difficulties from inhaling spores from mold |
| 14 | Salmonella | Getting food poisoning from Salmonella |
| 15 | Lightning | Being struck by lightning |
| 16 | Norovirus | Catching a bug which causes nausea, vomiting, diarrhea and abdominal cramping. Sometimes called the winter vomiting bug |
Table 3.
Ratio-scaled fear and control scores (RSS, sum to 100) with 95% confidence intervals.
| Risk | Fear | Control | Control (anchored)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RSS | 95% Lower |
95% Upper |
RSS | 95% Lower |
95% Upper |
RSSA | 95% Lower |
95% Upper |
|||
| Terrorist attack | 8.439 | 7.980 | 8.898 | 1.706 | 1.450 | 1.962 | − 29.595 | − 32.328 | − 26.863 | ||
| Dementia | 12.643 | 12.304 | 12.982 | 1.692 | 1.472 | 1.911 | − 24.027 | − 26.637 | − 21.416 | ||
| Car accident | 7.750 | 7.398 | 8.103 | 6.367 | 5.997 | 6.737 | 10.793 | 8.297 | 13.289 | ||
| Lung disease | 9.217 | 8.920 | 9.513 | 3.321 | 3.122 | 3.521 | − 3.831 | − 6.042 | − 1.621 | ||
| Antibiotic resistance | 6.196 | 5.864 | 6.529 | 4.510 | 4.257 | 4.762 | − 0.063 | − 2.320 | 2.195 | ||
| Fire | 7.869 | 7.466 | 8.273 | 12.210 | 11.860 | 12.559 | 37.436 | 34.953 | 39.919 | ||
| Diabetes | 4.794 | 4.520 | 5.067 | 9.519 | 9.192 | 9.846 | 21.964 | 19.409 | 24.519 | ||
| Heart attack | 13.496 | 13.256 | 13.735 | 5.249 | 4.920 | 5.579 | 6.809 | 4.314 | 9.303 | ||
| Pesticide residues | 2.882 | 2.668 | 3.096 | 6.616 | 6.312 | 6.921 | 5.571 | 3.137 | 8.006 | ||
| Skin cancer | 10.992 | 10.683 | 11.302 | 7.529 | 7.140 | 7.918 | 15.286 | 12.606 | 17.967 | ||
| Common cold | 0.548 | 0.409 | 0.687 | 6.926 | 6.495 | 7.356 | 10.262 | 7.321 | 13.202 | ||
| Dog bite | 1.341 | 1.142 | 1.539 | 9.794 | 9.424 | 10.163 | 18.412 | 15.827 | 20.998 | ||
| Bioaerosols | 3.527 | 3.329 | 3.725 | 5.736 | 5.534 | 5.938 | 3.129 | 0.911 | 5.347 | ||
| Salmonella | 3.857 | 3.617 | 4.097 | 9.348 | 9.075 | 9.622 | 20.007 | 17.456 | 22.557 | ||
| Lightning | 1.848 | 1.607 | 2.090 | 3.944 | 3.578 | 4.310 | − 12.750 | − 15.711 | − 9.789 | ||
| Norovirus | 4.601 | 4.288 | 4.914 | 5.533 | 5.239 | 5.827 | 5.285 | 2.827 | 7.743 | ||
The control scores are presented as unanchored (RSS) and anchored (RSSA) scores.
aZero = threshold for taking action to reduce the risk.
Figure 2.
The ranking of ratio-scaled scores (RSS) relating to (A) fear and (B) control for the 16 risk items (n = 806). Error bars represent 95% confidence intervals. Red striped bar shows risk associated with norovirus and orange, crosshatched bars show other environment-associated risks.
Respondents’ perceptions of control
Respondents believed they had the greatest levels of control over fire at home, dog bites, developing diabetes and suffering food poisoning from Salmonella (Fig. 2B). Norovirus was ranked 10th with the perceived level of control very similar to that for bioaerosols (i.e. breathing difficulties from inhaling spores from mold). Developing lung disease from air pollution ranked 14th as the least controlled environmental risk. The level of control people felt they had over norovirus was 70% lower than the perceived level of control over Salmonella food poisoning and 20% lower that the level of control over becoming ill from pesticide residues. People believed they had far greater (72%) levels of control over acquiring diabetes than they did over norovirus.
The consideration thus far has been relative—the degree of fear and control has been considered in relation to other risks included in the survey. The mixed logit model was re-estimated incorporating the information on which risks respondents took action to lower their risk (i.e. they exerted some control) with threshold normalized to zero (Fig. 3; Table 3). The anchored results indicate that norovirus is one of the risks people typically think they are able to control, as they do with illness from pesticide residues and bioaerosols. The marked difference in perceived control between norovirus and both diabetes and Salmonella is present in the anchored (RSSA) results. Getting dementia or developing lung disease are the two illness risks for which people did not take actions to lower their risk and hence scored below the zero threshold—along with ‘events’, such as lightning strike and terrorist attack.
Figure 3.
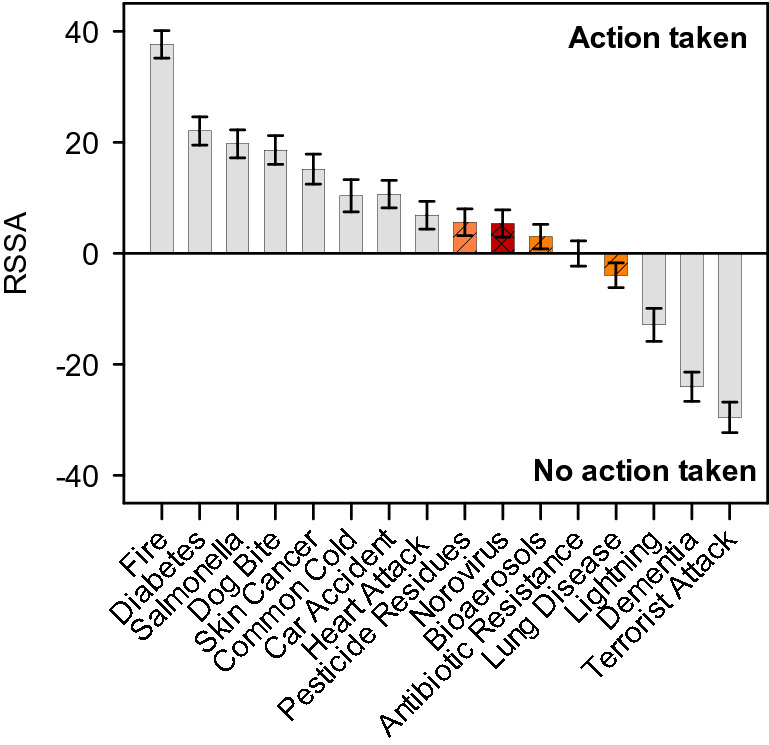
Anchored ratio-scaled control scores (RSSA). Zero = threshold for taking action to reduce the risk. The error bars represent 95% confidence intervals. Red striped bar shows risk associated with norovirus and orange, crosshatched bars show other environment-associated risks.
The results reported thus far used the mean fear and control scores for each risk, generated by estimation of the mixed logit model. The model also yielded individual level estimates, conditional on their control BWS choice data and the population parameters. These estimates allowed us to consider sample level heterogeneity and systematic differences in perceptions of control, over observable characteristics. This revealed differences between age groups, genders, education levels and between those who had, and had not, sought advice on gastroenteritis (Figure S2). The analysis showed no trends or significant differences in the perception of individuals in different ethnic groups, employment status, having children or previously experiencing water- or shellfish-borne gastroenteritis.
Females perceived themselves to have higher levels of control than males for risks including norovirus, Salmonella, getting diabetes, illness from pesticide residues, skin cancer and illness from bioaerosols. People aged 18–24 perceived themselves to have lower control over suffering a heart attack in their lifetime than those aged 45–64.
All risks were considered more controllable among the higher educated population. The difference was significant for most risk items, except fire at home, getting the common cold, being bitten by a dog and being struck by lightning. Respondents who had sought advice on gastroenteritis believed they had more control on all the risks than those who did not. The differences were significant except for fire at home, getting diabetes, suffering a heart attack, getting skin cancer and getting breathing difficulties due to bioaerosols.
Fear vs. control
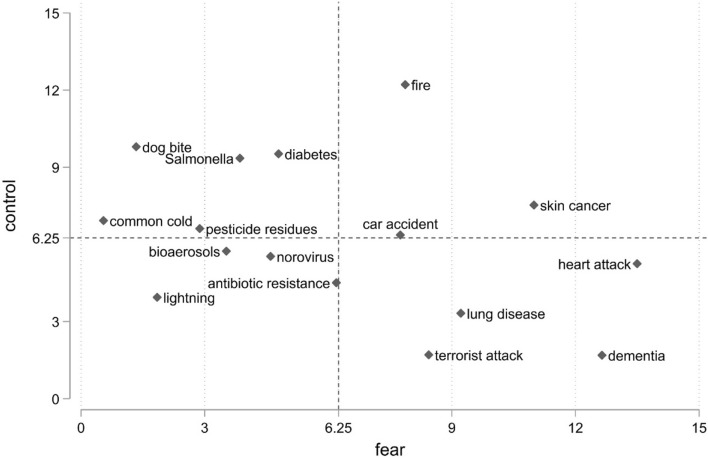
The results from the fear and (unanchored) control models are combined in Fig. 4 which shows the ratio-scaled fear and control scores (from Table 3). The re-scaling sums the scores to 100 and the axes represent mean levels of fear (and control) which, with 16 hazards included, is equal to score 6.25 (Fig. 4). Hence, risks ‘north’ of the x-axis indicate above average levels of control, and those ‘east’ of the y-axis indicate above average levels of fear. There was a negative correlation between the risk dimensions with higher levels of fear associated with lower levels of control. Risks, such as dementia, terrorist attack, having a heart attack or developing lung disease from air pollution, were associated with relatively high levels of fear and relatively low levels of control.
Figure 4.
Rescaled (0–100) mean fear and control ratio-scaled scores for the 16 risk items (n = 806) in 2-dimensional space. The scaling of scores was changed to sum up to 100 with the mean of 6.25.
Salmonella was characterized by relatively low fear levels and high levels of control. Illness from bioaerosols and pesticide residues were associated with similar levels of fear as Salmonella, but significantly lower levels of control. Norovirus generated higher levels of fear and less control than these three hazards.
Discussion
This study evaluated the public’s level of awareness of norovirus infection in relation to both environmental and general risks using an online survey. In order to identify the most vulnerable populations, only people regularly exposed to environmental risks (i.e. recreational water users and shellfish eaters) were included. It is estimated that 51% of the UK population consumes shellfish41, suggesting that the survey covered a great proportion of the population. However, there is no data available on people’s recreational activities.
Our study revealed that 28% of the respondents had gastroenteritis-related symptoms after using recreational water. Jones et al.42 have also found that 20% of people had diarrhea, 12% had nausea and 4% vomited up to three weeks after exposure to recreational water at Swansea, UK. In an UK-wide survey, Fleisher et al.43 also found that 14.9% of participants had gastroenteritis after bathing in natural waters. In both cases, the rates of illnesses were significantly higher among people exposed to water than in the control groups suggesting strong association between bathing and developing symptoms.
Our study revealed that participants had gastroenteritis after being in direct contact with a sick person (22%) and eating shellfish (16%), suggesting that these routes may also be significant routes of gastroenteritis transmission. Becoming ill from shellfish or recreational activities caused significant changes in people’s behavior; most people started to take precautions after being sick, such as increased use of hand sanitizers or no longer eating raw or lightly cooked shellfish.
The survey revealed the resources people used, or would use, to learn more about gastrointestinal diseases. We found, that although detailed information on the transmission and prevention of norovirus, and other environment-associated risks, is available on governmental websites, 74% of respondents were not aware of those resources. Understanding how and why people (fail to) become aware of the availability of such resources, could improve reduction and management of the risks. Improved understanding of those processes would be particularly valuable in relation to those groups most vulnerable to the risks.
Many respondents claimed to have good knowledge of some of the most common food- and waterborne pathogens, e.g. Salmonella and norovirus (Fig. 1). Their claimed levels of knowledge regarding norovirus and its synonym, ‘winter vomiting bug’, were very similar, suggesting that both terms are familiar to people. Norovirus is responsible for approx. 600,000 to one million cases each year in the UK44, hence the recognition of the pathogen was expected. Participants claimed to have far less knowledge on Listeria, a food pathogen with the most severe typical impact on health (a typical loss per case of 4.03 Quality Adjusted Life Years (QALYs) compared to 0.67 for norovirus45. Participants also claimed little knowledge of Campylobacter, even though this pathogen is the most common foodborne pathogen in the UK with over 300,000 cases and the highest annual burden on the economy (£424.4 M), followed by norovirus (£248.5 M) and Salmonella (£143.9 M)45 . Furthermore, rotavirus was not recognized by 48% of respondents. Rotavirus had been the most common cause of gastroenteritis among children under five years old until the vaccination introduced in 2013, which lowered the number of cases requiring hospitalization by 83%46. A similar decline in cases of Salmonella was observed between 2000 and 2017 due to improved legislation, food safety advice, and an industry-led flock vaccination program47, however, the number of lab-confirmed cases is still approx. 9000/year48. As all these foodborne pathogens cause mild gastroenteritis in the majority of cases, people may not be familiar with the different causative agents, and may believe their symptoms are caused by a pathogen they are familiar with (e.g. Salmonella, norovirus). Therefore, more information should be disseminated for people on the different kinds of pathogens. With that knowledge, people would be better able to assess their symptoms and more likely to seek medical help when necessary.
The main aim of this study was to investigate perceptions of norovirus compared to both other emerging risks associated with contaminated air and water and more established risks (e.g. heart attack, hit by lightning). The results of the BWS revealed low levels of fear and control regarding norovirus and other emerging environmental health risks compared to more general hazards. A possible reason may be that some of the risks, such as norovirus or Salmonella infection, mostly cause mild illness that resolve without treatment within days10,49.
Norovirus fear levels were equal to those from diabetes. This is remarkable given that over three million people in the UK have diabetes with this number estimated to rise to 4.6 million people by 203050. Norovirus prompted significantly higher levels of fear than Salmonella, pesticide residues and bioaerosols. This correlates with the number of cases and economic burden of the two foodborne pathogens45. The levels of fear from lung disease and antibiotic resistance were higher than the level of fear prompted by norovirus. In contrast, reverse association was observed in terms of control (Fig. 2; Table 3). This suggests that along with the severity of symptoms, active management (i.e. control) of a risk also influence the overall perception of the risk. With more knowledge on the precautions one can take to avoid certain hazards, the fear prompted by the hazard may be reduced. Therefore, taking into account the high number of norovirus cases each year, the lack of concern may be due to misinformation or the lack of information on the transmittance and prevention of the disease.
The results show significant gender differences in the perceived levels of control when anchored analysis was used. In general, women believed they had more control over the risks. However, many previous studies showed that women have similar or lower levels of control than men on a wide range of risks, such as being run over/burgled, stressed environment (including technological, behavioral, and land use hazards) and earthquakes51–54. We found the highest differences between men and women in the control of risks associated with food preparation (getting diabetes, becoming ill due to pesticides or Salmonella/norovirus infection) and personal hygiene and health (developing skin cancer, common cold and antibiotic resistance pathogens). In these areas, women may believe they are more informed on the risks and take measures to reduce them by for instance thoroughly washing vegetables, adequately cooking meat and cleaning and washing hands regularly55,56.
Our results also showed that the level of risk perception in terms of control increases with age and educational qualifications. These findings suggest that people are more aware of the risks with more experience and knowledge. We found no significant differences in the control levels of people with different employment status, family background, income or ethnicity. Many studies have explored the effect of socio-demographic factors on behavior, e.g. disaster preparedness or consumer behavior57–59, however, little information is available on the effect of these factors on risk perception. More targeted analysis is therefore necessary to explore the effect of socio-demographic factors on risk perception.
The BWS results suggest that those who sought advice on gastroenteritis perceived themselves to have higher levels of control over risks (related and unrelated) to the illness. That also indicates that people who sought advice are generally more aware and cautious of risks, and hence take measures to better understand and control them. As frequent exposure to messages on health and well-being have been shown to positively influence health-related behavior60, the targeted dissemination of information on environment-associated health risks may increase control over risk and hence reduce the number of illnesses. The information to be disseminated may include detailed information on the transmission of norovirus and options to mitigate risk of infection, e.g. hand washing, cooking shellfish and washing vegetables before consumption, good kitchen hygiene61. According to our analysis, men and people with low education levels believe they have the least control on environmental risks (Figure S2), hence distribution of information targeting these socio-demographic groups may be required. Our survey revealed that the information available on public health and food safety-related websites was regarded as adequate, however, many participants were not aware of these resources. Therefore, more information (including QR code and other links to official web resources) should be circulated at places where people would look for such advice, e.g. doctors’ offices, hospitals, medical centers and the health services’ websites. Albeit controversial, more information on food safety could also be placed on shellfish food packaging.
Conclusions
In this study, we assessed the public’s perception of norovirus infection in relation to other environment-associated and general health risks. Our results suggest that the level of norovirus awareness is relatively high compared to other foodborne pathogens (e.g. Listeria, Cryptosporidium, rotavirus or Campylobacter) and slightly lower than the claimed knowledge on Salmonella, E. coli and MRSA. Furthermore, our BWS results suggest that the levels of fear associated with norovirus are comparable to those from diabetes. Despite the considerable levels of awareness and fear, the level of control people felt they had over norovirus was low, significantly below that for Salmonella or diabetes.
Given the scale of the public health burden associated with norovirus, addressing this deficit in the public’s understanding of how to control exposure to the pathogen is an important public health need. However, our study revealed some association between perceived control on norovirus and age, gender and education. Further research is therefore needed to understand the effect of sociodemographic factors and behavior on the perception of norovirus and other food- and waterborne illnesses. Our current findings suggest that different approaches are needed to disseminate information regarding transmission of the virus to increase awareness and reduce risk of infection.
Methods
Survey design
The online survey we designed comprised of two sections. The first section elicited demographic information (age, gender, income, qualification, ethnicity etc.). The respondents were also asked about behaviors relevant to exposure to norovirus, their knowledge of the pathogen and their use of information sources related to it. Specifically:
Type and frequency of shellfish consumption.
How often, and where, they come into contact with recreational waters (including rivers, lakes and seawater in the UK).
Whether they had had contact with anyone with gastroenteritis or food/shellfish-related gastroenteritis over the past year and if so, where the shellfish was obtained.
Whether they had sought information about gastroenteritis over the past year and if so, whether they found the information useful.
The reason for not seeking information and where they would consider looking.
How much they had heard about certain enteric pathogens (Salmonella, methicillin-resistant Staphylococcus aureus—MRSA, Cryptosporidium, norovirus, Escherichia coli, Clostridium difficile, Listeria, winter vomiting bug, Shigella, Campylobacter, rotavirus) and a fictive one (Perginella), which acted as a control. We used the terms ‘norovirus’ and its synonym, ‘winter vomiting bug’, separately to assess which term is better known.
The survey referred to gastroenteritis as “upset stomach” defined as onset of stomach pains that can strike quickly with force and make a person feel very sick, which typically resolves within 2–3 days with symptoms of nausea, vomiting, diarrhea and abdominal cramping. The full list of questions is detailed in the Supplementary Material.
The second part of the survey comprised the Best Worst Scaling (BWS) exercise. BWS is a choice-based survey method designed to elicit the relative importance of multiple items enabling accurate ranking. Rather than asking respondents to rank large numbers of items, they are asked to choose the ‘most’ and ‘least’ from repeated subsets of items35. In our study, the items were the risks considered in the study (see Table 1) and respondents were asked to choose those they feared least and most and those they had most and least control over (Figure S1). The task was repeated with varying combinations of four items.
The BWS experiment contained 16 risks, including norovirus, other air, food and waterborne illnesses and other hazards (Table 1). Some of the risks were well-established in the risk perception literature (e.g. heart attack, car accident), whereas others were more novel (e.g. antibiotic resistance, bioaerosols). The allocation of risks into sets was determined by an experimental design which varied the combinations of risk presented, the frequency with which they occur and co-occur and their position in the sets (top, middle, bottom). Respondents completed 12 tasks using each criteria (fear, control) with the risks randomly allocated.
The approach employed here resembles that of Erdem and Rigby53, who elicited perceptions of a set of risks in terms of fear and control—as these had been identified in the psychometric risk perception literature as dimensions on which risks could be meaningfully located. We augmented their approach by the introduction of an absolute threshold into the analysis of control levels. The BWS method, as typically employed, is a relative assessment of the items under consideration. In this case, as well as eliciting the relative degree of fear that the hazards induced, and the degree of control people perceived they had over them, we also asked, for each risk, whether the respondent ever amended their behavior to reduce the risk. We then incorporated that threshold information into the analysis of control. Further details on survey and experiment design and model specification can be found in the Supplementary Material.
Data collection and analysis
The survey was distributed by an online marketing company (Research Now, UK). Only adults (over 18 years old) who had consumed bivalve shellfish and had been in contact with environmental waters within the previous year were included in the study. The survey was piloted on the 13th February 2018 with 100 participants. When the required number of answers was reached, the survey was paused, and survey responses were checked to ensure that sensible answers were received. Based on the responses, the skip logic was working, people saw the right responses based on their answers and the ratio of replies were what we predicted and people who did not meet the criteria were disqualified. The survey was then administered between the 15th and 20th February 2018 with 1006 participants. After the removal of incomplete responses, those who had completed the survey so quickly as to suggest inadequate consideration of their responses (n = 194), the sample comprised 806 responses.
The BWS data were analyzed via estimation of random utility models62 and mixed logit models63,64, as detailed in the Supplementary Material. Best–worst raw scores were calculated for each risk and then transformed into standardized ratio-scaled (0–100) scores (RSS) and anchored ratio-scaled scores (RSSA) incorporating threshold data using Lighthouse Studio65.
Ethical statement
The ethical approval for this study was obtained from the Ethics Committee of Bangor University. The online survey was handled by Research Now, UK. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. This manuscript does not contain any individual person’s data in any form. The introductory text of the survey informed the participants that the survey was part of a scientific project with its aims and methods explained, and that participation was optional. Informed consent for participation was obtained by Research now, UK.
Supplementary Information
Acknowledgements
This work was funded by the Natural Environment Research Council (NERC) under the Environmental Microbiology and Human Health (EMHH) Programme (NE/M010996/1) and by the Shellfish Centre RD&I operation, part-funded by the EU’s West Wales and the Valleys European Regional Development Fund (ERDF) Operational Programme through the Welsh Government. The authors wish to thank David Lees, Andrew Turner, Andrew Younger and David Walker (Centre for Environment, Fisheries and Aquaculture Science, UK) for their help constructing the survey.
Author contributions
Conceptualization, K.F., E.G., D.R., P.C., S.M., D.J., S.T.; Methodology, K.F., E.G., D.R., P.C., S.M., D.J., S.T.; Data analysis, K.F., E.G., D.R.; Writing—original draft preparation, K.F., E.G., D.R., P.C.; Writing—review & editing, K.F., E.G., D.R., P.C., S.M., D.J., S.T.; Funding acquisition, D.J., S.T.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kata Farkas and Emma Green.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90704-7.
References
- 1.Cohen AJ, et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Heal. Part A. 2005;68:1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- 2.Lin, W. E., Carpentieri, M. & Savory, E. Transmission of human respiratory disease by indoor bioaerosols. in Physmod 2017—International Workshop on Physical Modelling of Flow and Dispersion Phenomena, Dynamics of Urban and Coastal Atmosphere—LHEEA -École Centrale de Nantes, France 23–25 August 2017 (2017).
- 3.Kallawicha K, et al. Exposure to ambient bioaerosols is associated with allergic skin diseases in Greater Taipei residents. Environ. Pollut. 2016;216:845–850. doi: 10.1016/j.envpol.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 4.Trout D, Bernstein J, Martinez K, Biagini R, Wallingford K. Bioaerosol lung damage in a worker with repeated exposure to fungi in a water-damaged building. Environ. Health Perspect. 2001;109:641–644. doi: 10.1289/ehp.01109641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations, 2014. (2014). 10.1038/510015a.
- 6.Efstratiou A, Ongerth JE, Karanis P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2011–2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Thongprachum A, Khamrin P, Maneekarn N, Hayakawa S, Ushijima H. Epidemiology of gastroenteritis viruses in Japan: Prevalence, seasonality, and outbreak. J. Med. Virol. 2016;88:551–570. doi: 10.1002/jmv.24387. [DOI] [PubMed] [Google Scholar]
- 8.Chatziprodromidou IP, Bellou M, Vantarakis G, Vantarakis A. Viral outbreaks linked to fresh produce consumption: A systematic review. J. Appl. Microbiol. 2018;124:932–942. doi: 10.1111/jam.13747. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SM, et al. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014;14:725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama, H. & Vinje, J. Norovirus and other Caliciviruses. in Global Water Pathogen Project (eds. Meschke, J. S. & Gironés, R.) (Michigan State University, E. Lansing, MI, UNESCO, 2017).
- 11.Barardi CR, et al. Monitoring viruses in environmental samples. Int. J. Environ. Sci. Eng. Res. 2012;3:62–79. [Google Scholar]
- 12.CDC. Norovirus worldwide. (2016). (Accessed 29 May 2019). https://www.cdc.gov/norovirus/worldwide.html.
- 13.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global economic burden of norovirus gastroenteritis. PLoS ONE. 2016;11:1–16. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellou M, Kokkinos P, Vantarakis A. Shellfish-borne viral outbreaks: A systematic review. Food Environ. Virol. 2013;5:13–23. doi: 10.1007/s12560-012-9097-6. [DOI] [PubMed] [Google Scholar]
- 15.Radin D. New trends in food- and waterborne viral outbreaks. Arch. Biol. Sci. 2014;66:1–9. doi: 10.2298/ABS1401001R. [DOI] [Google Scholar]
- 16.Aoki Y, et al. Duration of norovirus excretion and the longitudinal course of viral load in norovirus-infected elderly patients. J. Hosp. Infect. 2010;75:42–46. doi: 10.1016/j.jhin.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu JPS, Sena K, Hodgers L, Palmer A, Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2017;616:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Y, et al. Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. J. Appl. Microbiol. 2015;119:1729–1739. doi: 10.1111/jam.12971. [DOI] [PubMed] [Google Scholar]
- 19.Kitajima M, Iker BC, Pepper IL, Gerba CP. Relative abundance and treatment reduction of viruses during wastewater treatment processes—Identification of potential viral indicators. Sci. Total Environ. 2014;488:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- 20.Iaconelli M, et al. One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food Environ. Virol. 2017;9:79–88. doi: 10.1007/s12560-016-9263-3. [DOI] [PubMed] [Google Scholar]
- 21.Farkas K, et al. Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and estuarine receiving waters. Sci. Total Environ. 2018;634:1174–1183. doi: 10.1016/j.scitotenv.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Silverman AI, Nelson KL, Akrong MO, Amoah P, Drechsel P. Quantification of human norovirus GII, human adenovirus, and fecal indicator organisms in wastewater used for irrigation in Accra, Ghana. J. Water Health. 2013;11:473–488. doi: 10.2166/wh.2013.025. [DOI] [PubMed] [Google Scholar]
- 23.Hassard F, et al. Abundance and distribution of enteric bacteria and viruses in coastal and estuarine sediments—A review. Front. Microbiol. 2016;7:1692. doi: 10.3389/fmicb.2016.01692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landry EF, Vaughn JM, Vicale TJ, Mann R. Accumulation of sediment-associated viruses in shellfish. Appl. Environ. Microbiol. 1983;45:238–247. doi: 10.1128/AEM.45.1.238-247.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowther JA, Gustar NE, Powell AL, Hartnell RE, Lees DN. Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Appl. Environ. Microbiol. 2012;78:5812–5817. doi: 10.1128/AEM.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Deng Z. Modeling and prediction of oyster norovirus outbreaks along Gulf of Mexico Coast. Environ. Health Perspect. 2016;124:627–633. doi: 10.1289/ehp.1509764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacCannell T, et al. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect. Control Hosp. Epidemiol. 2011;32:939–969. doi: 10.1086/662025. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm. Reports. 2011;60:1–18. [PubMed] [Google Scholar]
- 29.Baert L, et al. Review: Norovirus prevalence in Belgian, Canadian and French fresh produce: A threat to human health? Int. J. Food Microbiol. 2011;151:261–269. doi: 10.1016/j.ijfoodmicro.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Viau EJ, Lee D, Boehm AB. Swimmer risk of gastrointestinal illness from exposure to tropical coastal waters impacted by terrestrial dry-weather runoff. Environ. Sci. Technol. 2011;45:7158–7165. doi: 10.1021/es200984b. [DOI] [PubMed] [Google Scholar]
- 31.Mara D, Sleigh A. Estimation of norovirus infection risks to consumers of wastewater-irrigated food crops eaten raw. J. Water Health. 2010;8:39–43. doi: 10.2166/wh.2009.140. [DOI] [PubMed] [Google Scholar]
- 32.Sano D, et al. Risk management of viral infectious diseases in wastewater reclamation and reuse: Review. Environ. Int. 2016;91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liverani M, et al. Understanding and managing zoonotic risk in the new livestock industries. Environ. Health Perspect. 2013;121:873–877. doi: 10.1289/ehp.1206001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anacleto P, Barrento S, Nunes ML, Rosa R, Marques A. Portuguese consumers’ attitudes and perceptions of bivalve molluscs. Food Control. 2014;41:168–177. doi: 10.1016/j.foodcont.2014.01.017. [DOI] [Google Scholar]
- 35.Finn A, Louviere JJ. Determining the appropriate response to evidence of public concern: The case of food safety. J. Public Policy Mark. 1992;11:12–25. doi: 10.1177/074391569201100202. [DOI] [Google Scholar]
- 36.Lin C-TJ, Milon JW. Attributes and safety perception in a double-hurdle model of shellfish consumption. Am. J. Agric. Econ. 1993;75:724–729. doi: 10.2307/1243579. [DOI] [Google Scholar]
- 37.Le Bihan V, Pardo S, Guillotreau P. Risk perception and risk management strategies of oyster farmers. Mar. Resour. Econ. 2013;28:285–304. doi: 10.5950/0738-1360-28.3.285. [DOI] [Google Scholar]
- 38.Borchardt MA, Spencer SK, Kieke BA, Lambertini E, Loge FJ. Viruses in nondisinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ. Health Perspect. 2012;120:1272–1279. doi: 10.1289/ehp.1104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edelstein M, Wallensten A, Zetterqvist I, Hulth A. Detecting the norovirus season in Sweden using search engine data—Meeting the needs of hospital infection control teams. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight AJ, Worosz MR, Todd ECD. Serving food safety: Consumer perceptions of food safety at restaurants. Int. J. Contemp. Hosp. Manag. 2007;19:476–484. doi: 10.1108/09596110710775138. [DOI] [Google Scholar]
- 41.Mintel. Executive summary: Fish and Shellfish. (2017).
- 42.Jones F, Kay D, Stanwell-Smith R, Wyer M. Results of the first pilot-scale controlled cohort epidemiological investigation into the possible health effects of bathing in seawater at Langland Bay, Swansea. Water Environ. J. 1991;5:91–98. doi: 10.1111/j.1747-6593.1991.tb00593.x. [DOI] [Google Scholar]
- 43.Fleisher JM, Kay D, Wyer MD, Godfree AF. Estimates of the severity of illnesses associated with bathing in marine recreational waters contaminated with domestic sewage. Int. J. Epidemiol. 1998;27:722–726. doi: 10.1093/ije/27.4.722. [DOI] [PubMed] [Google Scholar]
- 44.HPA. Norovirus Fact Sheet. (2011).
- 45.FSA. Estimating Quality Adjusted Life Years and Willingness to Pay Values for Microbiological Foodborne Disease (Phase 2). (2017).
- 46.Hungerford D, et al. Early impact of rotavirus vaccination in a large paediatric hospital in the UK. J. Hosp. Infect. 2016;93:117–120. doi: 10.1016/j.jhin.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien SJ. The, “decline and fall” of nontyphoidal salmonella in the United Kingdom. Clin. Infect. Dis. 2013;56:705–710. doi: 10.1093/cid/cis967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.FSA. Consolidated annual report and accounts 2017/18. (Food Standards Agency, 2018).
- 49.D’Aoust J-Y. Pathogenicity of foodborne Salmonella. Int. J. Food Microbiol. 1991;12:17–40. doi: 10.1016/0168-1605(91)90045-Q. [DOI] [PubMed] [Google Scholar]
- 50.Holman N, Forouhi NG, Goyder E, Wild SH. Epidemiology The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: Estimates of total diabetes prevalence for England, 2010–2030. Diabet. Med. 2011;28:575–582. doi: 10.1111/j.1464-5491.2010.03216.x. [DOI] [PubMed] [Google Scholar]
- 51.Greenberg MR, Schneider DF. Gender differences in risk perception: Effects differ in stressed vs. non-stressed environments. Risk Anal. 1995;15:503–511. doi: 10.1111/j.1539-6924.1995.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 52.Ming-Chou H, Daigee S, Shuyeu L, Yao-Chu C. How do disaster characteristics influence risk perception? Risk Anal. 2008;28:635–643. doi: 10.1111/j.1539-6924.2008.01040.x. [DOI] [PubMed] [Google Scholar]
- 53.Erdem S, Rigby D. Investigating heterogeneity in the characterization of risks using best worst scaling. Risk Anal. 2013;33:1728–1748. doi: 10.1111/risa.12012. [DOI] [PubMed] [Google Scholar]
- 54.Yi-Wen K, Sue-Huei C. Perception of earthquake risk in Taiwan: Effects of gender and past earthquake experience. Risk Anal. 2012;32:1535–1546. doi: 10.1111/j.1539-6924.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 55.Jay LS, Comar D, Govenlock LD. A National Australian food safety telephone survey. J. Food Prot. 2016;62:921–928. doi: 10.4315/0362-028X-62.8.921. [DOI] [PubMed] [Google Scholar]
- 56.Lake AA, et al. Food shopping and preparation among the 30-somethings: Whose job is it? (The ASH30 study) Br. Food J. 2006;108:475–486. doi: 10.1108/00070700610668441. [DOI] [Google Scholar]
- 57.Miceli R, Sotgiu I, Settanni M. Disaster preparedness and perception of flood risk: A study in an alpine valley in Italy. J. Environ. Psychol. 2008;28:164–173. doi: 10.1016/j.jenvp.2007.10.006. [DOI] [Google Scholar]
- 58.Verbeke W. Consumer acceptance of functional foods: Socio-demographic, cognitive and attitudinal determinants. Food Qual. Prefer. 2005;16:45–57. doi: 10.1016/j.foodqual.2004.01.001. [DOI] [Google Scholar]
- 59.Nunes F, Madureira T, Oliveira JV, Madureira H. The consumer trail: Applying best–worst scaling to classical wine attributes. Wine Econ. Policy. 2016;5:78–86. doi: 10.1016/j.wep.2016.10.002. [DOI] [Google Scholar]
- 60.Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet. 2010;376:1261–1271. doi: 10.1016/S0140-6736(10)60809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guix S, Pintó R, Bosch A. Final consumer options to control and prevent foodborne norovirus infections. Viruses. 2019;11:333. doi: 10.3390/v11040333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McFadden D. Conditional logit analysis of qualitative choice behavior. In: Zarembka P, editor. Frontiers in Econometrics. Academic Press; 1973. pp. 105–142. [Google Scholar]
- 63.Train K. Discrete Choice Methods with Simulation. Cambridge University Press; 2003. [Google Scholar]
- 64.McFadden D, Train K. Mixed MNL models of discrete response. J. Appl. Econom. 2000;15:447–470. doi: 10.1002/1099-1255(200009/10)15:5<447::AID-JAE570>3.0.CO;2-1. [DOI] [Google Scholar]
- 65.Sawtooth. Sawtooth Software. (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.