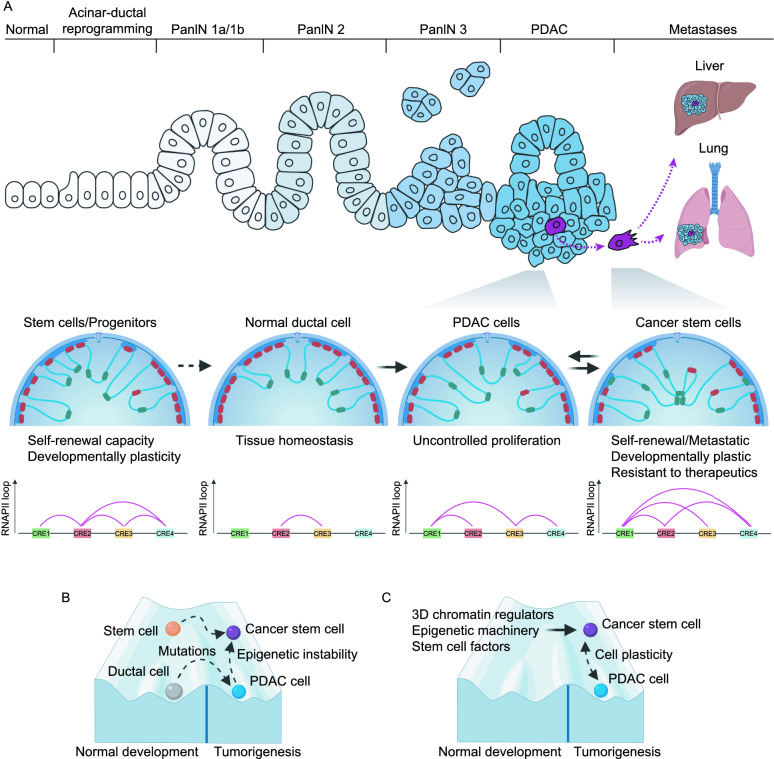
Figure 2.
Targeting 3D chromatin architecture in CSCs. (A) Rearrangement of the higher-order epigenome during tumorigenesis leads to changes in the cell state from differentiated cell to cancer cells and to cancer stem cells (CSCs). Pancreatic ductal adenocarcinoma (PDAC) formation occurs by gradual transitioning of normal ductal cells to pancreatic intraepithelial neoplasia (PanIN) lesions that ultimately leads to PDAC formation. The PDAC is a heterogenous cell population containing cancer stem cells (CSCs) that can self-renew, are developmental plasticity, highly metastatic and more resistant to conventional therapeutics than other cancer cells. The simplified depiction of the higher-order epigenome inside the nuclei during tumorigenesis indicating rearrangements of active (green) and inactive (red) chromatin regions as well as lamina-associated domains (blue). The genome is organized into topologically associated domains that form gene expression domains with enhancer-promoter crosstalk. Different chromatin regions are postulated to be brought together in different cell states such as CSCs by transcription factors and regulatory proteins that facilitate chromatin accessibility and gene expression regulation. The differences in cell states are postulated to manifest at the higher-order but also lower-order levels of chromatin that bind RNAPII and sequence-specific transcription factors (pink lines show interaction clusters), as depicted schematically by four cis-regulatory elements (CREs). (B) The Waddington landscape of normal tissue development and tumorigenesis. In normal tissue formation, stem cells or progenitor cells differentiated to genetically and epigenetically stable cell types, such as ductal cells in pancreatic tissue. Genomic instability due to the accumulation of mutations in driver genes, and epigenomic instability leads to the formation of CSCs and non-CSCs either from normal stem cells or differentiated ductal cells, or through further dedifferentiation of non-CSCs to CSCs. (C) The phenotypic plasticity of CSCs and non-CSCs depicted on the Waddington landscape. The plasticity of CSCs allows the cells to give rise to the whole tumour in its entire cellular heterogeneity while non-CSCs are less plastic. The plasticity is hypothetically regulated by the interplay between 3D epigenome, epigenetic machinery, stem cell factors and structural proteins that regulate gene expression