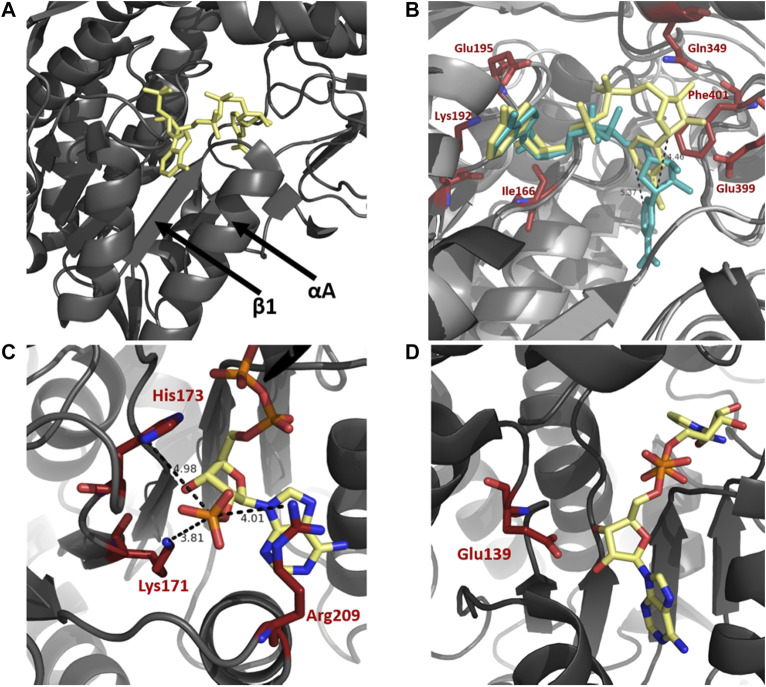
FIGURE 4.
Cofactor binding mechanism of ALDH. (A) Depiction of αA and β1 of the Rossmann fold in the cofactor binding domain. (B) Varying conformations of the nicotinamide portion and constant orientation of adenine ring of NAD demonstrated on sheep ALDH1 and bovine ALDH2 (PDB: 1BXS and 1A4Z). ALDH1 (dark gray) and ALDH2 (light gray) are modeled as an overlay with NAD in yellow and blue, respectively. Measurements demonstrate an approximate 5 Å shift of the nicotinamide portion. Key cofactor binding residues are highlighted in red. Residues 241–253 have been omitted for visualisation purposes. (C) NADP 2′phophate shown in close proximity to Lys171, His173, and Arg209, no acidic residue is present in the NADP dependent ALDH from V. Harvei (PDB: 1EYY). (D) Human ALDH3A1 (PDB: 4L2O), a non-obligatory NADP ALDH, shows a NAD cofactor in close proximity to Glu139 even though this ALDH can utilise NADP.