Abstract
The novel coronavirus disease 2019 (COVID-19) has become a matter of international concern as the disease is spreading exponentially. Statistics showed that infected patients in China who received combined treatment of Traditional Chinese Medicine and modern medicine exhibited lower fatality rate and relatively better clinical outcomes. Both Lian-Hua-Qing-Wen Capsule (LHQWC) and Jin-Hua-Qing-Gan Granule (JHQGG) have been recommended by China Food and Drug Administration for the treatment of COVID-19 and have played a vital role in the prevention of a variety of viral infections. Here, we desired to analyze the broad-spectrum anti-viral capacities of LHQWC and JHQGG, and to compare their pharmacological functions for rational clinical applications. Based on literature mining, we found that both LHQWC and JHQGG were endowed with multiple antiviral activities by both targeting viral life cycle and regulating host immune responses and inflammation. In addition, from literature analyzed, JHQGG is more potent in modulating viral life cycle, whereas LHQWC exhibits better efficacies in regulating host anti-viral responses. When translating into clinical applications, oral administration of LHQWC could be more beneficial for patients with insufficient immune functions or for patients with alleviated symptoms after treatment with JHQGG.
Keywords: broad-spectrum antivirals, Lian-Hua-Qing-Wen capsule, Jin-Hua-Qing-Gan granule, medicinal plants, COVID-19, SARS-CoV-2, host-directed therapy
Introduction
Lian-Hua-Qing-Wen Capsule and Jin-Hua-Qing-Gan Granule are Both Recommended as Effective “Chinese Solution” Against COVID-19
The novel coronavirus disease 2019 (COVID-19) pandemics has reached almost every country in the world. Compared with the outbreak of Severe Acute Respiratory Syndrome (SARS) in 2003 and the pandemic of Middle East Respiratory Syndrome (MERS) in 2012, COVID-19 caused by the novel coronavirus SARS-CoV-2 infection has relatively low fatality rate, whereas much more rapid and higher human-to-human transmissibility (Meo et al., 2020). Typically, the existence of a large number of asymptomatic carriers of SARS-CoV-2 additionally exerts potential burden to the control and prevention of COVID-19.
SARS-CoV-2 can be easily transmitted through respiratory droplets or by aerosol, and infected people have a wide range of reported symptoms, from mild symptoms to severe illness. The most common manifestations of COVID-19 are fever or chill, dry cough and fatigue, which could be accompanied with a temporary loss of smell or taste, muscle or body aches. In critical cases, acute myocardial injury, liver or kidney dysfunction and blood-clotting complications may occur Huang et al. (2020), Khider et al. (2020), consequently leading to septic shock and acute respiratory distress syndrome (ARDS) or death. The “Clinical Treatment for COVID-19” issued by the World Health Organization recommends that symptomatic treatments that relieve fever and pain, together with adequate nutritional supports are basically required for mild cases of COVID-19. For severe SARS-CoV-2 infections, oxygen therapy and fluid supply need to be reinforced. In spite of supportive measures above, potential anti-viral drugs which were used for diseases due to viral infections other than SARS-CoV-2 have been repurposed for COVID-19, such as remdesivir, ribavirin and hydroxychloroquine are however not addressed because of reported side-effects or lack of supporting evidence from large-scale randomized controlled trials (Izcovich et al., 2020; Trivedi et al., 2020; Qaseem et al., 2021). Likewise, vaccine development involves a difficult, complex and costly process, and the success of which is at a high risk of failure protecting against mutant viral variants (Biswas and Majumder, 2020; Penarrubia et al., 2020). Despite the development of vaccines, scientists are still tirelessly designing new drugs and repurposing existing drugs against SARS-CoV-2. Though tremendous strides have been made in the fight against coronaviruses, a lack of safe and effective anti-SARS-CoV-2 drugs is still a key factor restricting the prevention and control of COVID-19 pandemics.
The practice of Traditional Chinese Medicine (TCM) has accumulated a wealth of clinical experience in the treatment of infectious diseases since Qin-Han (about 221 BC to 220 AD) and developed into a theory in Ming-Qing period (about 1,368–1777 AD). Infectious diseases in TCM have been described as “infections caused by toxic qi”, “warm pathogen first invades lung via nose and mouth”, and “disease spreads due to close contact”. These descriptions fit well with the epidemiological characteristics of modern acute infectious diseases. According to TCM theory, COVID-19 is the result of invasion by dampness-toxin pathogens, therefore COVID-19 is pathogenically characterized by dampness-toxin and host healthy-qi deficiency. Most patients first present mild sign of dampness, like fatigue, poor appetite and greasy thick tongue coating (Zheng, 2020). As disease progresses, dampness-toxin invades interiority and diffuses into triple energizer, leading to vital qi impairment and accumulation of toxin-qi in viscera. Excessive accumulation of dampness-toxin may easily lead to vital qi exhaustion and consequently loss of life. Hence, TCM formulae functioning to remove dampness-toxin are effective in preventing COVID-19 progress. Being the first country that was attacked by COVID-19, approximately 91.5% confirmed patients in China were treated with TCM formulae and the total effective rate has reached to 90%. In Wuhan Jiang-Xia Square Cabin Hospital, none of the 564 COVID-19 patients who received combined treatment of TCM and modern medicine developed into severe conditions, and TCM addition significantly reduced the course of hospitalization (Ren et al., 2020).
Both LHQWC and JHQGG belong to “Three Drugs, Three Prescriptions”, official prescriptions of TCM used in the fight against COVID-19 in China. LHQWC, composed of Forsythia suspensa (Thunb.) Vahl, Lonicera japonica Thunb., honey-fried Ephedra sinica Stapf, fried Prunus sibirica L., Gypsum Fibrosum, Isatis tinctoria L., Dryopteris crassirhizoma Nakai, Houttuynia cordata Thunb., Pogostemon cablin (Blanco) Benth., Rheum palmatum L., Rhodiola crenulata (Hook.f. and Thomson) H. Ohba, Mentha canadensis L. and Glycyrrhiza glabra L., is innovative Chinese Patent Medicine (CPM) approved during the SARS epidemics in 2003. JHQGG, the other CPM constituting Forsythia suspensa (Thunb.) Vahl, Lonicera japonica Thunb., Ephedra sinica Stapf, Prunus sibirica L., l-Menthol, Glycyrrhiza glabra L., Scutellaria baicalensis Georgi, Fritillaria thunbergii Miq., Anemarrhena asphodeloides Bunge, Arctium lappa L. and Artemisia annua L., has been approved to treat H1N1 influenza virus infection since 2009. Both LHQWC and JHQGG are developed based on Ma-Xing-Shi-Gan Decoction and Yin-Qiao Powder, classic TCM decoctions used for respiratory infections recorded in Treatize on Exogenous Febrile Disease (about 210 AD) and Systematic Differentiation of Warm Diseases (1798 AD), respectively. In clinical practices resolving respiratory infections, LHQWC is mainly used to clear away plague, remove toxins, ventilate lungs and discharge heat, whereas JHQGG is applied to dispel wind, clear heat and resolve toxin. In the combat against COVID-19, National Health Commission of China approved both LHQWC and JHQGG as clinical therapies in China, and observational studies showed that both can effectively relieve fever, fatigue, cough and phlegm in the early stage of COVID-19, contributing to reductions in risks of rapid clinical deterioration. Supportively, in vitro studies have revealed that both formulae have anti-inflammatory effects, providing fundamental evidence for clinical application of both formulae in the fight against COVID-19 (Cheng, 2020; Duan, 2020; Hu et al., 2020; Runfeng et al., 2020; Zhang et al., 2020).
Holism Theory of TCM and Anti-viral Actions of Lian-Hua-Qing-Wen Capsule and Jin-Hua-Qing-Gan Granule, a Reflection of Host-Directed Therapy in Modern Medicine
Holism is the fundamental concept in TCM, which emphasizes the connections of the whole body and intends to treat the whole person rather than focusing on individual symptoms. Directed by holistic view, TCM practitioners adopt syndrome differentiation (Bian Zheng), a comprehensive analysis of a variety of clinical information, and herbal formulae to resolve single or complex uncomfortability of patients. This holism theory of TCM dovetails with the principle of host-directed therapy (HDT). HDT is a novel concept in the treatment for infectious diseases and was first used in tuberculosis in 2015 (Zumla et al., 2015). After then, HDT was gradually fulfilled as anti-viral strategies. Compared to conventional anti-viral therapies, which focus on inhibiting virus activity, HDT aims to maintain homeostasis of host by stimulating anti-viral responses and suppressing immune injuries. It has been shown that compared to single anti-pathogen treatment, HDT is able to reduce the risks of drug resistance induced by bacteria and viruses, endowing HDT a therapeutic potential of being broad-spectrum anti-viral tactics (Kaufmann et al., 2018). Clinical investigations proposed that viral infection-triggered cytokine storm was a vital factor mediating the rapid progress of COVID-19 (Wang T. et al., 2020). High levels of IL (Interleukin) -6 and IL-10, while low levels of CD4+ T and CD8+ T cells can be observed in COVID-19 patients (Guan et al., 2020; Wan et al., 2020). Moreover, plasma IL-2, IL-7, IL-10, GCSF (granulocyte colony-stimulating factor), IP-10 (interferon gamma-induced protein-10), MCP-1 (monocyte chemoattractant protein-1), MIP-1α (macrophage inflammatory protein-1 alpha) and TNF-α (tumor necrosis factor-alpha) are consistently higher in intensive care unit (ICU) patients compared to mild cases (Huang et al., 2020), suggesting that virus-induced exaggerated immune responses and the resulting immune injuries are involved in the progression of COVID-19. Accordingly, HDT-oriented treatments that inhibit IL-6 signaling by down-regulating IL-6 receptors have been suggested as a potential solution for COVID-19 patients (Zumla et al., 2020). Consistent with HDT, in the combat against COVID-19, TCM addresses that sufficient healthy-qi within the body is key to prevent pathogen invasion, so-called “strengthening host resistance to eliminate pathogenic factors”. Accordingly, inspiring vital qi is at the root of preventing infectious diseases in TCM. The functions of “healthy-qi” resemble “immunity” of host, and “pathogenic factors” stand for all substances that affect host homeostasis, such as viruses and bacteria. As emphasized in HDT that considering individuals as a whole rather than separating parts, “strengthening host resistance to eliminate pathogenic factors” in TCM addresses an overall reaction of host in response to invasive viruses, whereas the destiny of pathogen itself is not primarily important. Moreover, same as the HDT concept implicates, the ultimate goal of TCM treatment is to maintain host homeostasis via balancing interactions between host and pathogens, or by establishing equilibrium between stimulating anti-viral reactions and suppressing overactivated immune responses that subsequently cause tissue injuries.
Following the HDT principle and holism theory of TCM, this study primarily desired to gain more insight into the broad anti-viral features of LHQWC and JHQGG, both of which have been applied to treat a variety of viral infections. However, considering that the main herbal composition of LHQWC and JHQGG largely overlap, it therefore appears confusing in the selection of appropriate formula for individual clinical cases. In this scenario, it is of prime importance to also distinguish the similarities and differences between the two formulae in terms of pharmacological anti-viral functions. To implement these goals, we manually grouped the individual active components from either LHQWC or JHQGG or both into two categories, namely constituents that interfere with viral life cycle and components that regulate host immune responses and inflammation. Through comprehensive literature review, data mining and pharmacological target enrichment analysis, we investigated the strength of LHQWC and JHQGG in the above-mentioned virus or host arm to compare their anti-viral functionalities. The holism-directed analysis of LHQWC and JHQGG will provide more insightful information and comprehensive understanding for rational use of these two CPMs in the combat against COVID-19, as well as the emerging or re-emerging pandemics of infectious diseases.
Materials and Methods
Literature Collection and Inclusion
In order to collect sufficient data on anti-viral effects of LHQWC and JHQGG, we employed Pubmed (https://pubmed.ncbi.nlm.nih.gov), Ovid (https://ovidsp.ovid.com/), CNKI (https://www.cnki.net), WANFANG (http://www.wanfangdata.com.cn/index.html) and WEIPU (http://www.cqvip.com/) database by searching either the full name of formulae, such as “Lianhua Qingwen Capsules”, “Jinhua Qinggan Granules”, or names of individual medicinal herbs, or active ingredients, together with “virus” as keywords. In addition, bioactive components that were proposed to be antivirals were included via network pharmacology-based prediction and analysis. A total of 1,110 articles were collected for next filtration. For the analysis of broad anti-viral activities, we then excluded studies reporting negative outcomes, clinical trials generally indicating viral infections without clarifying taxonomy of viruses, investigations using inactivated or attenuated viruses as vaccines, and articles with no access to full context due to age. A total of 812 articles were analyzed at this stage. For detailed comparisons of active anti-viral components and pharmacological functions of formulae, studies without indicating names of active components were further excluded. Notably, no information regarding Gypsum Fibrosum and fried Prunus sibirica L. in relevant to virus, and we did not find data by searching bioactive components directly isolated from JHQGG, hence we only took ingredients determined by predictive parsing of network pharmacology. Finally, 117 articles were included for comparison of pharmacological functions.
Constructing “Formula–Herb–Virus–Baltimore Classification of Viruses” Network
In order to describe broad-spectrum anti-viral activities of LHQWC and JHQGG, we grouped antiviral data collected as mentioned, and built a network in forms of “Formula-herb-virus-Baltimore classification of viruses”. To further interpret the common and distinctive anti-viral activities of LHQWC and JHQGG in terms of holism theory of TCM, we classified the anti-viral actions reported for LHQWC and JHQGG into being either associated with viral life cycle or responsible to host immune responses and inflammation. To gain more insightful understanding, we further categorized active components that disrupt virus life cycle into three levels, including direct virucidal activity, inhibition of viral entry, and suppression of viral replication and egress. Generally, inhibitors of virus entry act through deforming viral particles or blocking the attachment or binding of virions to host cells. The control of virus replication is mainly mediated by inhibiting replicator machineries encoded by viral systems, and prevention of virus egress is a process involves an interference with assembly and release of progeny viruses, which may initiate a secondary round infection. For the actions of regulating host immune responses and inflammation, it represents any virucidal effects due to an indirect response by modulating host immune system, such as increasing interferons (IFNs) expression, or decreasing self-targeted inflammatory injuries, or promoting repair process post virus infection without involving viral molecule-associated biological events. Based on literature mining and analysis, we next counted the frequencies of active components of LHQWC and JHQGG that have been sorted into each of the two categories, and accordingly a radar chart was drawn to visualize and compare the power of LHQWC and JHQGG against viral infection in terms of modulating viral life cycle and regulating host immune responses and inflammation.
Results
The broad-Spectrum Anti-Viral Activities of Lian-Hua-Qing-Wen Capsule and Jin-Hua-Qing-Gan Granule
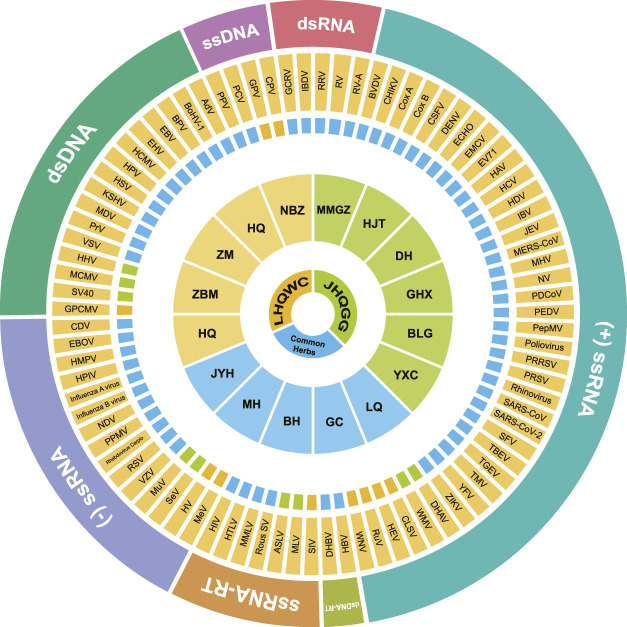
Multi-ingredients, multi-targets and multi-pathways are primary features of TCM formulae, suggesting that active ingredients of one medicinal herb may exert anti-viral functions via diverse pharmacological mechanisms. As shown in Figure 1, active components in both LHQWC and JHQGG have been shown to target 87 different types of viruses, covering all the seven classes according to the Baltimore classification. This wide range of anti-viral activities of LHQWC and JHQGG addresses that TCM formulae used in COVID-19 pandemics could be potentially applied for other virological infections, such as influenza A virus, Zika virus and herpesvirus.
FIGURE 1.
The broad-spectrum anti-viral activities of LHQWC and JHQGG. The “Formula–herb–virus–Baltimore classification of viruses” profile demonstrating a broad-spectrum anti-viral activity of LHQWC and JHQGG. In the center, medicinal herbals exclusively existing in LHQWC, including HQ (Scutellaria baicalensis Georgi, Huang Qin); ZBM (Fritillaria thunbergii Miq., Zhe Bei Mu); ZM (Anemarrhena asphodeloides Bunge, Zhi Mu); QH (Artemisia annua L., Qing Hao) and NBZ (Arctium lappa L., Niu Bang Zi) are shown in orange; medicinal herbals found only in JHQGG, including MMGZ (Dryopteris crassirhizoma Nakai, Mian Ma Guan Zhong); HJT (Rhodiola crenulata (Hook.f. and Thomson) H. Ohba, Hong Jing Tian); DH (Rheum palmatum L., Da Huang); GHX (Pogostemon cablin (Blanco) Benth., Guang Huo Xiang); BLG (Isatis tinctoria L., Ban Lan Gen) and YXC (Houttuynia cordata Thunb., Yu Xing Cao); are presented in green; common herbs used in both LHQWC and JHQGG, including LQ (Forsythia suspensa (Thunb.) Vahl, Lian Qiao); GC (Glycyrrhiza glabra L., Gan Cao); BH (Mentha canadensis L., Bo He); MH (Ephedra sinica Stapf, Ma Huang) and JYH (Lonicera japonica Thunb., Jin Yin Hua) are colored in blue. The circle marked in orange represents 87 types of viruses, and the cycle in the periphery indicates Baltimore classification of these viruses. Colored squares sitting between the circle of individual herbs and 87 viruses indicate that components existing only in LHQWC (orange) or only in JHQGG (green) or in both formulae (blue) have been reported effective to treat diseases caused the corresponding viruses. AdV, Adenoviruses; ASLV, Avian sarcoma leukosis virus; BoHV, Bovine alphaherpesvirus; BPV, Bovine papillomavirus; BVDV, Bovine viral diarrhea virus; CDV, Canine distemper virus; CHIKV, Chikungunya virus; CLSV, Cucumber leaf spot virus; Cox A, Coxsackie A virus; Cox B, Coxsackie B virus; CPV, Canine parvovirus; CSFV, Classical swine fever virus; DENV, Dengue virus; DHAV, Duck hepatitis A virus; DHBV, Duck hepatitis B virus; EBOV, Ebola virus; EBV, Epstein–Barr virus; ECHO, Echovirus; EHV, Equine herpes virus; EMCV, Encephalomyocarditis virus; EV71, Enterovirus A 71; GCRV, Grass carp reovirus; GPCMV, Guinea pig cytomegalovirus; GPV, Goose parvovirus; HAV, Hepatitis A virus; HBV, Hepatitis B virus; HCMV, Human cytomegalovirus; HCV, Hepatitis C virus; HDV, Hepatitis D virus; HEV, Hepatitis E virus; HHV, Human herpesvirus; HIV, Human immunodeficiency virus; HMPV, Human metapneumovirus; HPIV, Human parainfluenza virus; HPV, Human papillomavirus; HSV, Herpes simplex virus; HTLV, Human T lymphotropic virus; HV, Hantavirus; IBDV, Infectious bursal disease virus; IBV, Infectious bronchitis virus; JEV, Japanese encephalitis virus; KSHV, Kaposi's sarcoma herpesvirus; MCMV, Murine cytomegalovirus; MDV, Marek's disease virus; MERS-CoV, Middle East respiratory syndrome coronavirus; MHV, Mouse Hepatitis virus; MLV, Murine leukemia virus; MMLV, Moloney Murine Leukemia virus; MuV, Mumps virus; NDV, Newcastle disease virus; NV, Norovirus; PCV, Porcine circovirus; PDCoV, Porcine deltacoronavirus; PEDV, Porcine epidemic diarrhea virus; PepMV, Potato–Pepino mosaic virus; PPV, Porcine parvovirus; PPMV, pigeon paramyxovirus; PPV, Pigeonpox virus; PRRSV, Porcine reproductive and respiratory syndrome virus; PRSV, Papaya ringspot virus; PrV, Pseudorabies virus; Rous SV, Rous sarcoma virus; RRV, Ross River virus; RSV, Respiratory syncytial virus; RuV, Rubella virus; RV, Rotavirus; RV-A, SA-11 Simian rotavirus; SARS-CoV, Severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SeV, Sendai virus; SFV, Semliki Forest virus; SIV, Simian immunodeficiency virus; SV40, Simian virus 40; TBEV, Tick-borne encephalitis virus; TGEV, Transmissible Gastroenteritis virus; TMV, Tobacco mosaic virus; VSV, Vesicular stomatitis virus; VZV, Varicella zoster virus; WMV, Watermelon mosaic virus; WNV, West Nile virus; YFV, Yellow fever virus; ZIKV, Zika virus. RNA, Ribonucleic Acid; -ssRNA, Negative-sense single-strand RNA; +ssRNA, Positive-sense single-stranded RNA; dsRNA, Double-stranded RNA; ssRNA-RT, Single-stranded RNA virus-reverse transcriptase; DNA, Deoxyribonucleic Acid; ssDNA, Single-stranded DNA; dsDNA, Double-stranded DNA; dsDNA-RT, Double-stranded DNA virus-reverse transcriptase.
Similarities and Differences of Lian-Hua-Qing-Wen Capsule and Jin-Hua-Qing-Gan Granule as Antivirals
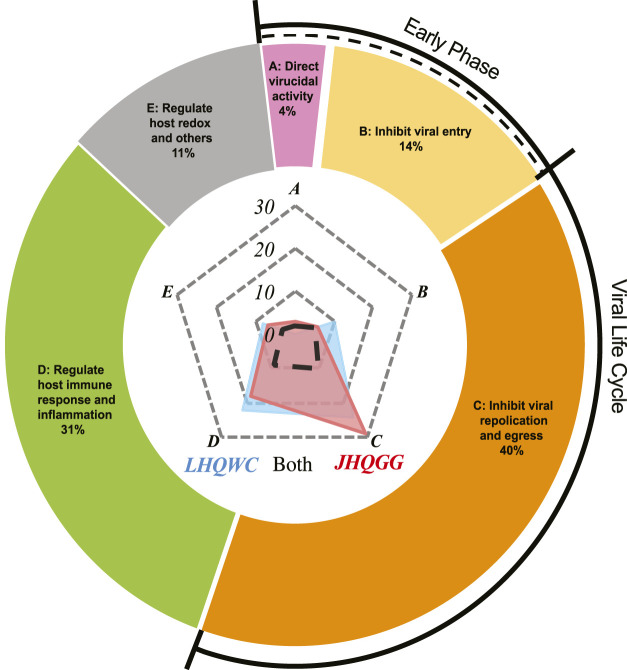
Both LHQWC and JHQGG possess broad-spectrum anti-viral potentials through interfering with viral life cycle and modulating host immune responses, which are associated with a diversity of proposed pharmacological actions as detailed in Tables 1, 2, 3; Figure 2. When comparing LHQWC and JHQGG, no difference was found in the types of their targeted viruses (Table 1; Figure 1). In terms of active components that disrupt viral life cycle (Table 1; Figure 2), only few literatures reported a direct virucidal activity from components of LHQWC and JHQGG (Table 1-1.1; Figure 2), about 24% studies showed suppression of viral entry (Table 1-1.2; Figure 2), while 70% studies focused on inhibitory effects toward viral replication and release (Table 1-1.3; Figure 2) Among all data analyzed, constituents from Scutellaria baicalensis Georgi (Huang Qin) of JHQGG have been mostly reported to interfere with viral life cycle in all three phases analyzed. Besides, components from Isatis tinctoria L (Ban Lan Gen) and Rheum palmatum L (Da Huang) of LHQWC are shown highly effective in blocking viral entry, replication and release. JHQGG weights slightly higher than LHQWC in terms of viral replication and release, whereas little difference was obtained in the early phase of viral life cycle (Table 1; Figure 2). Regarding “host immune responses and inflammation”, it is interesting that constituents from Scutellaria baicalensis Georgi (Huang Qin) of JHQGG again exhibited the greatest potential, followed by components from Isatis tinctoria L (Ban Lan Gen) and Rheum palmatum L (Da Huang) in LHQWC. When comparing LHQWC and JHQGG, LHQWC weights slightly higher than JHQGG (Table 2; Figure 2). In addition, several studies have proposed other anti-viral mechanisms that could not be grouped into the above two categories, such as maintaining host redox homeostasis, or acting on microbiota, or gut-lung axis, or energy sensor AMPK, or autophagy (Table 3; Figure 2). Detailed information regarding the TCM features, pharmacological functions of individual herbs and components was outlined in Table 4.
TABLE 1.
Active anti-viral components from LHQWC and JHQGG, and their mechanisms of action regulating viral life cycle.
| 1.1 Direct virucidal activity | |||
|---|---|---|---|
| Virus | Active component | Herb | References |
| Chikungunya Virus | Baicalin | Scutellaria baicalensis Georgi (Huang Qin) | Oo et al. (2018) |
| Coxsackievirus A16 | Glycyrrhizic acid | Glycyrrhiza glabra L. (Gan Cao) | Wang et al. (2013) |
| Herpes simplex virus type1 | Chinonin/Asphonin | Anemarrhena asphodeloides Bunge (Zhi Mu) | Jiang and Xiang (2004) |
| Newcastle disease virus | Baicalin | Scutellaria baicalensis Georgi (Huang Qin) | Jia et al. (2016) |
| Respiratory syncytial virus | Lonicera japonica Thunb extracts | Lonicera japonica Thunb. (Jin Yin Hua) | Zhang et al. (2014) |
| 1.2 Inhibit viral entry | ||||
|---|---|---|---|---|
| Virus | Active component | Mechanisms | Herb | Ref |
| Coxsackie virus B3 | Artemisinin | Inhibits viral absorption | Artemisia annua L. (Qing Hao) | Ma (2004) |
| Baicalin | Reduces cellular lipid synthesis | Scutellaria baicalensis Georgi (Huang Qin) | Wang et al. (2020a) | |
| Herpes simplex virus | Houttuynia cordata Thunb. Extracts | Blocks viral binding and penetration | Houttuynia cordata Thunb. (Yu Xing Cao) | Zhou (2017); Hung et al. (2015) |
| Herpes simplex virus type1 | Isatis tinctoria L. extracts | Inhibits viral entry | Isatis tinctoria L. (Ban Lan Gen) | Fang, 2005) |
| Herpes simplex virus type1 type2 and varicella zoster virus | Houttuynoid A | Blocks viral membrane fusion | Houttuynia cordata Thunb. (Yu Xing Cao) | Li et al. (2017a) |
| Herpes simplex virus type2 | Chinonin/Asphonin | Inhibits viral adsorption | Anemarrhena asphodeloides Bunge (Zhi Mu) | Jiang et al. (2005) |
| Human cytomegalovirus | Baicalein | Blocks viral entry through inhibiting epidermal growth factor receptor tyrosine kinase activity and viral nuclear translocation | Scutellaria baicalensis Georgi (Huang Qin) | Evers et al. (2005) |
| Human rotavirus | Rheum palmatum L. extracts | Inhibits viral entry | Rheum palmatum L. (Da Huang) | He et al. (2013) |
| Influenza A Virus | Flavonoids-enriched extract from Scutellaria baicalensis root | Reduces hemagglutinin | Scutellaria baicalensis Georgi (Huang Qin) | Zhi et al. (2019) |
| Rhein | Inhibits viral absorption | Rheum palmatum L. (Da Huang) | Wang et al. (2018) | |
| Isatis tinctoria L. extract Clemastanin B, epigoitrin, phenylpropanoids portion and the mixture of phenylpropanoids, alkaloids and organic acid fractions | Blocks viral attachment | Isatis tinctoria L. (Ban Lan Gen) | Xiao et al. (2016) | |
| Glycyrrhizin | Reduces endocytotic activity and virus uptake | Glycyrrhiza glabra L. (Gan Cao) | Wolkerstorfer et al. (2009) | |
| Isatis tinctoria L. water extracts | Inhibits attachment of viruses to cells | Isatis tinctoria L. (Ban Lan Gen) | Chen et al. (2006) | |
| (+)-catechin | Inhibits acidification of endosomes and lysosomes | Ephedra sinica Stapf (Ma Huang) | Mantani et al. (2001) | |
| 5,7,4′-trihydroxy-8-methoxyflavone | Inhibits fusion of virus with endosome/lysosome membrane | Scutellaria baicalensis Georgi (Huang Qin) | Nagai et al. (1995a); Nagai et al. (1995b) | |
| Influenza A virus, Coxsackievirus B3, Adenovirus | Patchouli alcohol | Inhibits infection at the earliest stages of the viral life cycle, including virus attachment and entry | Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) | Wei et al. (2013) |
| Porcine reproductive and respiratory syndrome virus | Flavaspidic acid AB | Inhibits viral endocytosis | Dryopteris crassirhizoma Nakai (Mian Ma Guan Zhong) | Yang et al. (2013) |
| Respiratory syncytial virus | Lonicera japonica Thunb. Extracts | Inhibits viral absorption | Lonicera japonica Thunb. (Jin Yin Hua) | Zhang et al. (2014) |
| Ephedra Sinica water extracts | Inhibits viral absorption and penetration | Ephedra sinica Stapf (Ma Huang) | Zhu and Li (2012) | |
| Radix Glycyrrhizae water extracts | Inhibits viral attachment and penetration | Glycyrrhiza glabra L. (Gan Cao) | Yeh et al. (2013) | |
| SARS Coronavirus | Emodin | Targets spike glycoprotein thus inhibits receptor binding | Rheum palmatum L. (Da Huang) | Ho et al. (2007) |
| 1.3 Inhibit viral replication and release | ||||
|---|---|---|---|---|
| Virus | Active component | Mechanisms | Herb | Ref |
| Bovine viral diarrhea virus, a surrogate in vitro model of hepatitis C virus | Novel artemisinin derivatives (AD) | AD1 and AD2 inhibit the release of Bovine viral diarrhea virus -RNA | Artemisia annua L. (Qing Hao) | Blazquez et al. (2013) |
| Coxsackie virus B3 | Emodin | Unknown | Rheum palmatum L. (Da Huang) | Cai and Luo (2014) |
| Artemisinin | Inhibits viral replication | Artemisia annua L. (Qing Hao) | Ma (2004) | |
| Isatis tinctoria L. polysaccharides extracts | Inhibits viral replication | Isatis tinctoria L. (Ban Lan Gen) | Zhang et al. (2009) | |
| Coxsakievirus B5 and respiratory syncytial virus | Emodin | Inhibits Viral biological synthesis | Rheum palmatum L. (Da Huang) | Liu et al. (2015) |
| Dengue virus | Lonicera japonica Thunb. aqueous extracts | The microRNA let-7a targets viral non-structural protein1 | Lonicera japonica Thunb. (Jin Yin Hua) | Lee et al. (2017) |
| Ebola virus | 18β-glycyrrhetinic acid | Binds to nucleoprotein | Glycyrrhiza glabra L. (Gan Cao) | Fu et al. (2016) |
| Enterovirus 71 | Glycyrrhizic acid | Inhibits viral replication | Glycyrrhiza glabra L. (Gan Cao) | Wang et al. (2013) |
| Rheum palmatum L. extracts | Reduces viral replication | Rheum palmatum L. (Da Huang) | Lin et al. (2009) | |
| Norwogonin, oroxylin A, mosloflavone | Inhibits expression of viral capsid proteins | Scutellaria baicalensis Georgi (Huang Qin) | Choi et al. (2016) | |
| Baicalin | Interfers with 3D polymerase transcription and translation | Scutellaria baicalensis Georgi (Huang Qin) | Li et al. (2015) | |
| Honeysuckle-encoded microRNA2911 | Targets viral envelope protein1 gene of Enterovirus 71 | Lonicera japonica Thunb. (Jin Yin Hua) | Li et al. (2018) | |
| Emodin | Diminishes cell cycle arrest at S phase induced infection | Rheum palmatum L. (Da Huang) | Zhong et al. (2017) | |
| Epstein-Barr Virus | Baicalein | Represses Epstein–Barr nuclear antigen1 Q-promoter activity | Scutellaria baicalensis Georgi (Huang Qin) | Zhang et al. (2018) |
| 5,7,2′-trihydroxy- and 5,7,2′,3′-tetrahydroxyflavone | Unknown | Scutellaria baicalensis Georgi (Huang Qin) | Konoshima et al. (1992) | |
| Arctium lappa L. extracts | Suppresses viral replication and decreases viral antigen expression, including capsid antigen and early antigen | Arctium lappa L. (Niu Bang Zi) | Chen and Huang (1994) | |
| Hepatitis B virus | Novel artemisinin derivatives (AD) | AD1 and AD2 reduce the release of Hepatitis B virus -DNA | Artemisia annua L. (Qing Hao) | Blazquez et al. (2013) |
| Hepatitis C virus | Pheophytin | Inhibits Hepatitis C virus -nonstructural3 protease | Lonicera japonica Thunb. (Jin Yin Hua) | Wang et al. (2009a) |
| Herpes simplex virus | Houttuynia cordata Thunb. Extracts | Suppresses viral replication via inhibiting NF-κB activation | Houttuynia cordata Thunb. (Yu Xing Cao) | Hung et al. (2015) |
| Herpes simplex virus type1 | Isatis tinctoria L. extracts | Inhibits viral replication | Isatis tinctoria L. (Ban Lan Gen) | Fang (2005) |
| Arctium lappa L. hydroalcoholic extracts | Suppresses viral replication | Arctium lappa L. (Niu Bang Zi) | Dias et al. (2017) | |
| Chinonin/Asphonin | Inhibits viral replication | Anemarrhena asphodeloides Bunge (Zhi Mu) | Jiang and Xiang (2004) | |
| Herpes simplex virus type2 | Chinonin/Asphonin | Inhibits viral replication | Anemarrhena asphodeloides Bunge (Zhi Mu) | Jiang et al. (2005) |
| Human cytomegalovirus | Artemisinin-derived monomers artesunate (AS) | Inhibits viral replication as hypophosphorylation (activity) of the retinoblastoma protein (pRb) | Artemisia annua L. (Qing Hao) | Roy et al. (2015) |
| Genistein | Blocks viral immediate-early protein functioning | Scutellaria baicalensis Georgi (Huang Qin) | Evers et al. (2005) | |
| Human immunodeficiency virus type1 | Artemisia afra | Unknown | Artemisia annua L. (Qing Hao) | Lubbe et al. (2012) |
| Sennoside A | Inhibits viral replication by targeting viral reverse transcription process including inhibiting HIV-1 Reverse Transcriptase-associated DNA Polymerase and Ribonuclease H activities | Rheum palmatum L. (Da Huang) | Esposito et al. (2016) | |
| Baicalein | Binds to the hydrophobic region of the HIV-1 integrase catalytic core domain | Scutellaria baicalensis Georgi (Huang Qin) | Ahn et al. (2001) | |
| Baicalin | Inhibits HIV-1 reverse transcriptase activity | Scutellaria baicalensis Georgi (Huang Qin) | Kitamura et al. (1998) | |
| Containing Scutellaria baicalensis aqueous extracts | Inhibits human immunodeficiency virus type-1 protease | Scutellaria baicalensis Georgi (Huang Qin) | Lam et al. (2000) | |
| Human rotavirus | Rheum palmatum L. extracts | Inhibits viral replication | Rheum palmatum L. (Da Huang) | He et al. (2013) |
| Influenza A Virus | Isatis tinctoria L. erucic acid | Reduces viral polymerase transcription activity | Isatis tinctoria L. (Ban Lan Gen) | Liang et al. (2020) |
| Baicalein and biochanin A | Inhibits viral replication | Scutellaria baicalensis Georgi (Huang Qin) | Michaelis et al. (2014) | |
| Oroxylin A | Inhibits neuraminidase | Scutellaria baicalensis Georgi (Huang Qin) | Jin et al. (2018) | |
| Flavonoids-enriched extract from Scutellaria baicalensis root | Inhibits neuraminidase activities | Scutellaria baicalensis Georgi (Huang Qin) | Zhi et al. (2019) | |
| Baicalin | Inhibits RNA polymerase activity | Scutellaria baicalensis Georgi (Huang Qin) | Guo et al. (2016) | |
| Baicalin | Interacts with RNA binding domain of Non-structural protein1 | Scutellaria baicalensis Georgi (Huang Qin) | Nayak et al. (2014) | |
| Glycyrrhizin | Inhibits influenza virus polymerase activity | Glycyrrhiza glabra L. (Gan Cao) | Moisy et al. (2012) | |
| Aloe-emodin | Inhibits viral replication through galectin-3 up-regulation | Rheum palmatum L. (Da Huang) | Li et al. (2014) | |
| Baicalin | Inhibits viral replication | Scutellaria baicalensis Georgi (Huang Qin) | Sithisarn et al. (2013) | |
| Baicalin | Inhibits neuraminidase activity | Scutellaria baicalensis Georgi (Huang Qin) | Sithisarn et al. (2013) | |
| Isatis tinctoria L. extract Clemastanin B (CB), epigoitrin, phenylpropanoids portion (PEP) and the mixture of phenylpropanoids, alkaloids and organic acid fractions | Inhibits viral replication | Isatis tinctoria L. (Ban Lan Gen) | Xiao et al. (2016) | |
| Isatis tinctoria L. extracts | Suppresses expression of influenza virus nucleoprotein | Isatis tinctoria L. (Ban Lan Gen) | Xu et al. (2010) | |
| Pogostemon cablin (Blanco) Benth extracts | Suppresses viral replication | Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) | Yang (2010) | |
| Fritillaria thunbergii | Unknown | Fritillaria thunbergii Miq. (Zhe Bei Mu) | Kim et al. (2020) | |
| Chlorogenic acid | Inhibits neuraminidase | Lonicera japonica Thunb. (Jin Yin Hua) | Ding et al. (2017) | |
| Honeysuckle (HS)-encoded atypical microRNA-MIR2911 | Inhibits IAV-encoded PB2 and NS1 protein expression | Lonicera japonica Thunb. (Jin Yin Hua) | Zhou et al. (2015) | |
| Forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit | Reduces influenza viral M1 protein | Forsythia suspensa (Thunb.) Vahl (Lian Qiao) | Law et al. (2017) | |
| Chalcones | Inhibits neuraminidase activity | Glycyrrhiza glabra L. (Gan Cao) | Dao et al. (2011) | |
| Houttuynia cordata Thunb. flavonoids extracts | Inhibits neuraminidase activity | Houttuynia cordata Thunb. (Yu Xing Cao) | Ling et al. (2020) | |
| Isatis tinctoria L. N-butanol extracts | Inhibits viral replication | Isatis tinctoria L. (Ban Lan Gen) | Liu et al. (2012) | |
| Newcastle disease virus | Baicalin | Inhibits apoptosis of virus-infected cells and suppresses viral spread | Scutellaria baicalensis Georgi (Huang Qin) | Jia et al. (2016) |
| Polyphenolic extracts | Pogostemon cablin (Blanco) Benth polyphenolic extracts | Inhibits neuraminidase activity | Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) | Liu (2016) |
| Porcine epidemic diarrhea virus | Pogostemon cablin (Blanco) Benth polysaccharides extracts | Inhibits viral replication | Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) | Chen et al. (2020) |
| Porcine reproductive and respiratory syndrome virus | Isatis tinctoria L. polysaccharide extracts | Inhibits viral replication | Isatis tinctoria L. (Ban Lan Gen) | Wei et al. (2011) |
| Flavaspidic acid AB from Dryopteris crassirhizoma | Inhibits viral replication | Dryopteris crassirhizoma Nakai (Mian Ma Guan Zhong) | Yang et al. (2013) | |
| Isatis tinctoria L. polysaccharide extracts | Inhibits viral replication | Isatis tinctoria L. (Ban Lan Gen) | Liu (2016) | |
| Artemisinin | Inhibits viral replication | Artemisia annua L. (Qing Hao) | Liu (2016) | |
| Respiratory syncytial virus | Isatis root extract | Inhibits viral NS1 and L proteins | Isatis tinctoria L. (Ban Lan Gen) | Zhang (2017) |
| (-)-(R)-nyasol (= 4,4'-(1Z,3R)-Penta-1,4-diene-1,3-diyldiphenol and broussonin A | Unknown | Anemarrhena asphodeloides Bunge (Zhi Mu) | Bae et al. (2007) | |
| Lonicera japonica Thunb. Extracts | Inhibits viral biosynthesis | Lonicera japonica Thunb. (Jin Yin Hua) | Li (2010) | |
| SARS coronavirus | Houttuynia cordata Thunb. Extracts | Inhibits SARS-CoV 3C-like protease and RNA-dependent RNA polymerase | Houttuynia cordata Thunb. (Yu Xing Cao) | Lau et al. (2008) |
| Rheum palmatum L. extracts | Inhibits SARS coronavirus 3C-like protease | Rheum palmatum L. (Da Huang) | Luo et al. (2009) | |
TABLE 2.
Active anti-viral components from LHQWC and JHQGG regulating host immune responses and inflammation.
| Virus | Active component | Mechanisms | Herb | References |
|---|---|---|---|---|
| Bovine viral diarrhea virus | Forsythoside A | Promotes peripheral blood mononuclear cell proliferation and T cell activation, TRAF2-dependent CD28-4-1BB signaling; induces IFN-γ | Forsythia suspensa (Thunb.) Vahl (Lian Qiao) | Li et al. (2011) |
| Coxsackie virus B3 | Emodin | Reduces pro-inflammatory cytokines | Rheum palmatum L. (Da Huang) | Cai and Luo (2014) |
| Emodin | Regulates IL-17/IL-23 axis | Rheum palmatum L. (Da Huang) | Jiang et al. (2014) | |
| Rhodiola | Unknown | Rhodiola crenulata (Hook.f. and Thomson) H.Ohba (Hong Jing Tian) | Liu et al. (2002) | |
| Coxsakievirus B5 and respiratory syncytial virus | Emodin | Decreases IFN-α, enhance TNF-γ | Rheum palmatum L. (Da Huang) | Liu et al. (2015) |
| Hepatitis B virus | Isatis tinctoria L. polysaccharide extracts | Enhances IFN-α and antiviral proteins, including p-STAT-1, p-STAT-2, p-JAK1, p-TYK2, OAS1, and Mx, via activation of JAK/STAT signal pathway | Isatis tinctoria L. (Ban Lan Gen) | Wang et al. (2020b) |
| Hepatitis C virus | Artemisia annua polysaccharides | Promotes IFN-γ secretion | Artemisia annua L. (Qing Hao) | Bao et al. (2015) |
| Herpes simplex virus type1 | Essential oil of Mentha suaveolens | Unknown | Mentha canadensis L. (Bohe) | Civitelli et al. (2014) |
| Influenza A Virus | Isatis tinctoria L.erucic acid | Reduces viral RNA-induced pro-inflammatory mediators through inactivation of NF-κB and p38 MAPK signaling pathway, Reduce CD8 (+) cytotoxic T lymphocyte recruitment | Isatis tinctoria L. (Ban Lan Gen) | Liang et al. (2020) |
| Oroxylin A | Increases IFN-β and IFN-γ | Scutellaria baicalensis Georgi (Huang Qin) | Jin et al. (2018) | |
| Flavonoids-enriched extract from Scutellaria baicalensis root | Reduces TNF-α, IL-6 and MCP-1, increases IFN-γ and IL-10 | Scutellaria baicalensis Georgi (Huang Qin) | Zhi et al. (2019) | |
| Baicalin | Modulates non-structural protein1-mediated cellular innate immune responses, IFN-induced antiviral signaling and a decrease in PI3K/Akt signaling | Scutellaria baicalensis Georgi (Huang Qin) | Nayak et al. (2014) | |
| Phillyrin | Decreases IL-6 | Forsythia suspensa (Thunb.) Vahl (Lian Qiao) | Qu et al. (2016) | |
| Aloe-emodin | Restores NS1-inhibited STAT1-mediated antiviral responses | Rheum palmatum L. (Da Huang) | Li et al. (2014) | |
| Ephedra alkaloids: L-ephedrine and D-pseudo- ephedrine | Regulating TLRs and RIG-1 pathways | Ephedra sinica Stapf (Ma Huang) | Wei et al. (2019) | |
| Radix Isatidis extract | Promotes T, B lymphocytes | Isatis tinctoria L. (Ban Lan Gen) | Jin (2007) | |
| Radix Isatidis polysaccharides | Promotes IFN-γ secretion | Isatis tinctoria L. (Ban Lan Gen) | Zuo (2008) | |
| Salidroside | Reduces IL1-β, IL-6, TNF-α and CRP, increases the number of CD4 (+) T cells | Rhodiola crenulata (Hook.f. and Thomson) H.Ohba (Hong Jing Tian) | Lin (2020) | |
| Baicalin | Balances host inflammatory response to limit immunopathologic injury; downregulated the key factors of the RLRs signaling pathway | Scutellaria baicalensis Georgi (Huang Qin) | Pang et al. (2018) | |
| Baicalin | Inhibits TLR7/MyD88 signaling pathway | Scutellaria baicalensis Georgi (Huang Qin) | Wan et al. (2014) | |
| Biochanin A | Reduces AKT, ERK 1/2 and NF-kB | Scutellaria baicalensis Georgi (Huang Qin) | Sithisarn et al. (2013) | |
| Biochanin A | Inhibits IL-6, IL-8 and IP-10 | Scutellaria baicalensis Georgi (Huang Qin) | Sithisarn et al. (2013) | |
| Baicalin | Inhibits IL-6 and IL-8 | Scutellaria baicalensis Georgi (Huang Qin) | Sithisarn et al. (2013) | |
| Radix Isatidis polysaccharides | Suppresses pro-inflammatory IL-6 and chemokines (IP-10, MIG, and CCL-5), inhibits host TLR3 Signaling | Isatis tinctoria L. (Ban Lan Gen) | Li et al. (2017b) | |
| Wogonin | Reduces inflammatory factors | Scutellaria baicalensis Georgi (Huang Qin) | Wu (2011) | |
| Epigoitrin | Reduces mitochondria mitofusin-2, which elevated mitochondria antiviral signaling and subsequently increased IFN-β and interferon inducible transmembrane 3 (IFITM3) | Isatis tinctoria L. (Ban Lan Gen) | Luo et al. (2019) | |
| Rhein | Activates TLR4, Akt, p38, JNK MAPK, and NF-κB signal pathways | Rheum palmatum L. (Da Huang) | Wang et al. (2018) | |
| Baicalin | Reduces TNF-α,IL-1 and 5-HT; increases IFN-γ | Scutellaria baicalensis Georgi (Huang Qin) | Li (2019) | |
| Isatis tinctoria L.extracts | Regulates immune response by enhancing proliferation and function of T and B cells | Isatis tinctoria L. (Ban Lan Gen) | Jin (2007) | |
| Dryocrassin ABBA | Decreases bronchoalveolar lavage fluid pro-inflammatory cytokines, including IL-6, TNF-α, and IFN-γ, and increases anti-inflammatory cytokines, including IL-10 and MCP-1 | Dryopteris crassirhizoma Nakai (Mian Ma Guan Zhong) | Ou et al. (2015) | |
| Baicalin | Imcreases IFN-γ production | Scutellaria baicalensis Georgi (Huang Qin) | Chu et al. (2015) | |
| Lonicera Japonica Thunb polysaccharide | Increases IFN-γ | Lonicera japonica Thunb. (Jin Yin Hua) | Jia (2018) | |
| Lonicera Japonica water decoction | Increases IFN-γ | Lonicera japonica Thunb. (Jin Yin Hua) | Zhu (2016) | |
| Lonicerae Japonicae Los and Forsythiae Fructus | Modulates MMP pathway and PRKCA pathway | Lonicera japonica Thunb. (Jin Yin Hua) | Li (2017) | |
| Forsythoside A | Reduces TLR7, MyD88 and NF-κB p65 protein; Inducing Th1/Th2 differentiats toward Th2, and the Th17/Treg cells differentiates toward Treg | Forsythia suspensa (Thunb.) Vahl (Lian Qiao) | Deng et al. (2016) | |
| Ethanol extracts of Forsythia suspensa Vahl. (Oleaceae), Strobilanthes cusia (Ness.) O. Kuntze (Acanthaceae), Glycyrrhiza uralensis Fischer. (Leguminosae) | Suppresses RANTES secretion | Forsythia suspensa (Thunb.) Vahl (Lian Qiao) Isatis tinctoria L. (Ban Lan Gen) Glycyrrhiza glabra L. (Gan Cao) | Ko et al. (2006) | |
| Houttuynia cordata Thunb. flavonoids extracts | Inhibits TLR signaling, increases IFN-β, decreases of TLR3/4/7 and NF-κB p65(p), MCP-1), IL-8, TNF-α and MDA | Houttuynia cordata Thunb. (Yu Xing Cao) | Ling et al. (2020) | |
| Influenza A Virus and Influenza B Virus | Wogonin | Increases IFN | Scutellaria baicalensis Georgi (Huang Qin) | Seong et al. (2018) |
| Japanese encephalitis virus | Arctigenin | Anti-inflammatory | Arctium lappa L. (Niu Bang Zi) | Swarup et al. (2008) |
| Porcine reproductive and respiratory syndrome virus | Flavaspidic acid AB | Induces IFN-α, IFN-β, and IL1-β expression in porcine alveolar macrophages | Dryopteris crassirhizoma Nakai (Mian Ma Guan Zhong) | Yang et al. (2013) |
| Respiratory Syncytial Virus | Baicalin | Increases IFN-1, decreases IL-6, IL-12 | Scutellaria baicalensis Georgi (Huang Qin) | Zhang (2018) |
| Rhein | Inhibits NLRP3 inflammasome activation through NF-kB pathway | Rheum palmatum L. (Da Huang) | Shen et al. (2020) | |
| 4(3H)-Quinazolone | Inhibits IFN-β secretion | Isatis tinctoria L. (Ban Lan Gen) | He et al. (2017) | |
| Total alkaloids, lignans and organic acids of Radix Isatidis extracts | Regulates IFNβ, synergistic effects through RIG-I and MDA5 signaling pathways | Isatis tinctoria L. (Ban Lan Gen) | Xu et al. (2019) | |
| Baicalin joint resveratrol | Increase serum TNF-α, IL-2, IFN-γ and SIgA in bronchoalveolar lavage fluid | Scutellaria baicalensis Georgi (Huang Qin) | Cheng et al. (2014) | |
| Radix Glycyrrhizae water extracts | Induces IFN-β secretion | Glycyrrhiza glabra L. (Gan Cao) | Yeh et al. (2013) | |
| SARS coronavirus | Houttuynia cordata Thunb. Extract | Immunomodulatory effects: stimulating mouse splenic lymphocytes the proliferation and increasing the proportion of CD4 (+) and CD8 (+) T cells, increases secretion of IL-2 and IL-10 by mouse splenic lymphocytes | Houttuynia cordata Thunb. (Yu Xing Cao) | Lau et al. (2008) |
| Vesicular stomatitis virus | Extract from Scutellaria baicalensis containing baicalein and wogonin | Inhibits IFN-alpha and IFN- γ, and stimulates TNF-α and IL (IL-12, IL-10) production | Scutellaria baicalensis Georgi (Huang Qin) | Blach-Olszewska et al. (2008) |
| Baicalin | Increases IFN-γ, reduces TNF-α and IL-10 | Scutellaria baicalensis Georgi (Huang Qin) | Orzechowska et al. (2014) |
IFN, Interferon; IL, Interleukin; MCP-1 Monocyte chemoattractant protein-1; MDA5, Melanoma differentiation-associated protein 5; MIG, Monokine induced by gamma interferon; MMP, Matrix metalloproteinases; MYD88, Myeloid differentiation factor 88; NLRP3, NLR Family Pyrin Domain Containing 3; PRKCA, Protein Kinase C Alpha; RANTES, Regulated upon activation, normal T cell expressed and presumably secreted; RIG-I, Retinoic acid-inducible gene I; STAT, Signal transducer and activator of transcription; TLR, Toll-like receptor; TNF, Tumor Necrosis Factor; TRAF2, TNF Receptor-associated Factor 2; 5-HT, 5-hydroxytryptamine.
TABLE 3.
Active anti-viral components from LHQWC and JHQGG regulating host redox homeostasis and other molecular actions.
| 3.1 Regulate redox homeostasis | ||||
|---|---|---|---|---|
| Virus | Active component | Mechanisms | Herb | References |
| Herpes simplex virus type1 | Piperitenone oxide | Interferes with redox-sensitive cellular pathways for viral replication | Mentha canadensis L. (Bohe) | Civitelli et al. (2014) |
| Japanese encephalitis virus | Arctigenin | Promotes antioxidative effects | Arctium lappa L. (Niu Bang Zi) | Swarup et al. (2008) |
| Influenza A Virus | Oroxylin A | Activates the nuclear factor erythroid 2–related factor 2 (Nrf2) transcription to increase antioxidant activities | Scutellaria baicalensis Georgi (Huang Qin) | Ji et al. (2015) |
| Rhein | Reduces antioxidative stress | Rheum palmatum L. (Da Huang) | Wang et al. (2018) | |
| Coxsackie virus B3 | Emodin | Up-regulates anti-oxidant enzymes | Rheum palmatum L. (Da Huang) | Cai and Luo (2014) |
| Isatis tinctoria L. Salidroside | Increases myocardial SOD activity and decreases MDA | Isatis tinctoria L. (Ban Lan Gen) | Wang et al. (2009b) | |
| Honeysuckle | Inhibits oxidative stress | Lonicera japonica Thunb. (Jin Yin Hua) | Lou (2017) | |
| Porcine epidemic diarrhea virus | Pogostemon cablin (Blanco) Benth polysaccharides extracts | Increases SOD and GSH-Px activity and decreases MDA | Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) | Wang (2010) |
| Hepatitis C virus | A glycyrrhizin-containing preparation | Protects mitochondria against oxidative stress | Glycyrrhiza glabra L. (Gan Cao) | Korenaga et al. (2011) |
| 3.2 Other molecular actions | ||||
|---|---|---|---|---|
| Virus | Active component | Mechanisms | Herb | References |
| Enterovirus 71 | Baicalin | Inhibits virus-induced apoptosis through regulating the Fas/FasL signaling pathways | Scutellaria baicalensis Georgi (Huang Qin) | Li et al. (2015) |
| Influenza A Virus | Houttuynia cordata Thunb. polysaccharide extracts | Acts on intestine and microbiota | Houttuynia cordata Thunb. (Yu Xing Cao) | Chen et al. (2019) |
| Houttuynia cordata Thunb | Protects intestinal barrier and regulates mucosal immunity, which may be related to the regulation of gut-lung axis | Houttuynia cordata Thunb. (Yu Xing Cao) | Zhu et al. (2018) | |
| Baicalin | Reduces endothelin (ET-1) and ET-1 receptor | Scutellaria baicalensis Georgi (Huang Qin) | Wan (2015) | |
| Houttuynia cordata Thunb.polysaccharides | Regulates the balance of Th17/Treg cells in gut-lung axis | Houttuynia cordata Thunb. (Yu Xing Cao) | Shi et al. (2020) | |
| Influenza A Virus and influenza B Virus | Wogonin | Suppresses AMPK phosphorylation | Scutellaria baicalensis Georgi (Huang Qin) | Seong et al. (2018) |
| Human cytomegalovirus | Baicalin | Regulates vasoactive intestinal peptide | Scutellaria baicalensis Georgi (Huang Qin) | Qiao et al. (2013) |
| Artemisinin | Modulates cell cycle through CDKs and hypophosphorylation (activity) of the retinoblastoma protein (pRb) | Artemisia annua L. (Qing Hao) | Roy et al. (2015) | |
| Herpes simplex virus type 1 | Triterpene glycyrrhizic acid | Induces autophagy activator Beclin 1 to establish a resistance state to viral replication | Glycyrrhiza glabra L. (Gan Cao) | Laconi et al. (2014) |
GSH-Px, Glutathione peroxidase; MDA, Malondialdehyde; SOD, Superoxide dismutase.
AMPK, AMP-activated protein kinase; CDKs, Cyclin-dependent kinases; Th17/Treg, T helper 17 (Th17)/regulatory T cells (Tregs).
FIGURE 2.
Comparison of anti-viral mechanisms between LHQWC and JHQGG. Anti-viral potentials of LHQWC and JHQGG are grouped into five categories, which are defined as (A). Direct virucidal activity, (B). Inhibit viral entry, (C). Inhibit viral replication and egress, (D). Regulate host immune responses and inflammation and (E). Regulate host redox and others”. The percentage in each category indicates the power of both LHQWC and JHQGG in individual anti-viral actions, among which the “A. Direct virucidal activity” and “B. Inhibit viral entry” belong to the early phase of viral infection as marked by black dotted line; the “A. Direct virucidal activity”, “B. Inhibit viral entry” and “C. Inhibit viral replication and egress”together constitute the whole viral life cycle, as surrounded in black. Comparation of LHQWC and JHQGG is demonstrated in the center, with actions from components only in LHQWC shown in blue, only of JHQGG in red, and for both LHQWC and JHQGG are circled within the black dotted area. 0–40 represents counted frequencies of either LHQWC or JHQGG in each of the five categories.
TABLE 4.
Detailed information of TCM features and pharmacological functions of single medicinal herbs from LHQWC and JHQGG.
| 4.1 Specific medicinal herbs of LHQWC | |||||||
|---|---|---|---|---|---|---|---|
| Components of medicinal herbs | TCM properties | Key characteristics | Active component | Virus | Pharmacological functions | References | |
| Rheum palmatum L. (Da Huang) | Bitter | Purges clumped heat in the intestines | Emodin | Coxsackie virus B3 | Decreases overall mortality of virus-induced murine viral myocarditis model and potentially could act through inhibiting viral replication, reducing pro-inflammatory cytokines and up-regulation of anti-oxidant enzymes | Cai and Luo (2014) | |
| Cold | Removes blood stasis | Reduces mice mortality rate and ameliorates myocardial damage by regulating the IL-17/IL-23 axis | Jiang et al. (2014) | ||||
| Stops bleeding in its charred form | Coxsackie virus B5 | Inhibits activities against coxsackie virus B5 | Liu et al. (2015) | ||||
| Enterovirus 71 | Inhibits viral replication and diminishes cell cycle arrest at S phase induced by EV71 infection in MRC5 cells | Zhong et al. (2017) | |||||
| Aloe-emodin | Influenza A Virus | Inhibits viral replication through galectin-3 up-regulation | Li et al. (2014) | ||||
| Rhein | Respiratory syncytial virus | Suppresses lung inflammatory injury by reducing the release of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, IL-18, and IL-33, in the serum and lung tissues of RSV-induced BALB/c mice through inhibiting NLRP3 inflammasome activation via NF-κB pathway | Shen et al. (2020) | ||||
| Influenza A virus | Inhibits viral absorption | Wang et al. (2018) | |||||
| Sennoside A | Human immunodeficiency virus type1 | Inhibits the HIV-1 replication by targeting the HIV-1 reverse transcription process including inhibiting HIV-1 Reverse Transcriptase-associated DNA Polymerase and Ribonuclease H activities | Esposito et al. (2016) | ||||
| Extracts | SARS coronavirus | Inhibits SARS coronavirus 3C-like protease | Luo et al. (2009) | ||||
| Rotavirus | Inhibits viral entry and replication in MA-104 cells | He et al. (2013) | |||||
| Houttuynia cordata Thunb.(Yu Xing Cao) | Acrid | Disperses heat | Houttuynoid A | Herpes simplex virus type 1 | Exhibits strong antiviral activity including inhibiting viral replication, inactivating viral infectivity by blocking viral membrane fusion and preventing lesion formation in HSV-1 infection mouse model. It also exhibits antiviral activities against other alpha herpes viruses, such as HSV-2 and varicella zoster virus | Li et al. (2017a) | |
| Cool | Resolves toxicity | Polysaccharides extracts | Influenza A virus | Oral administration could ameliorate lung injury in virus-infected mice via directly regulating the balance of Th17/Treg cells in gut-lung axis | Shi et al. (2020) | ||
| Reduces swelling | Acts on intestine and microbiota | Chen et al. (2019) | |||||
| Flavonoids extracts | Influenza A virus | Significantly inhibit viral proliferation and suppress neuraminidase activity and TLR3, TLR4, and TLR7 agonist-stimulated cytokine secretion, NF-κB p65 phosphorylation, and nuclear translocation in vitro | Ling et al. (2020) | ||||
| Extracts | Influenza A virus | Protects intestinal barrier and regulates mucosal immunity, which may be related to the regulation of gut-lung axis | Zhu et al. (2018) | ||||
| Enterovirus 71 | Reduces plaque formation and neutralizes virus-induced cytopathic effects in Vero cells and could affect apoptotic processes in virus-infected Vero cells by inhibiting viral replication | Lin et al. (2009) | |||||
| SARS coronavirus | Exerts anti-viral effects, including inhibitory effects on SARS-CoV 3C-like protease and RNA-dependent RNA polymerase. Exhibits immunomodulatory effects, including stimulating the proliferation of mouse splenic lymphocytes and increasing the proportion of CD4 (+) and CD8 (+) T cells and the secretion of IL-2 and IL-10 by mouse splenic lymphocytes | Lau et al. (2008) | |||||
| Herpes simplex virus | Inhibits the infection of HSV-1, HSV-2, and acyclovir-resistant HSV-1 via blocking viral binding and penetration. Suppresses viral replication via inhibiting NF-κB activation | Hung et al. (2015) | |||||
| Isatis tinctoria L. (Ban Lan Gen) | Bitter | Drains heat | Erucic acid | Influenza A virus | Suppresses viral replication by reducing viral polymerase transcription activity and inhibits RNA-induced pro-inflammatory mediators through inactivation of NF-κB and p38 MAPK signaling pathway. Inhibits alveolar epithelial A549 cells apoptosis. Decreases lung viral load and viral antigens expression, and reduces CD8 (+) cytotoxic T lymphocyte recruitment, which results in decreasing lung injury and mortality of virus-infected mice | Liang et al. (2020) | |
| Cold | Resolves fire toxicity | Epigoitrin | Influenza A virus | Reduces mitochondria mitofusin-2, which elevated mitochondria antiviral signaling and subsequently increased IFN-β and interferon inducible transmembrane 3 | Luo et al. (2019) | ||
| Cools the blood | 4(3H)-Quinazolone | Respiratory Syncytial Virus | Inhibits IFN-β secretion | He et al. (2017) | |||
| Benefits the throat | Clemastanin B, epigoitrin, phenylpropanoids portion and the mixture of phenylpropanoids, alkaloids and organic acid fractions | Influenza A virus | Inhibits viral replication, entry and improves the viability of infected MDCK cells | Xiao et al. (2016) | |||
| Polysaccharide extracts | Influenza A virus | Inhibits virus replication and reduces the expression of pro-inflammatory cytokines (IL-6) and chemokines (IP-10, MIG, and CCL-5) by inhibiting TLR-3 signaling pathway activation | Li et al. (2017b) | ||||
| Hepatitis B virus | Reduce extracellular and intracellular level of HBsAg, HBeAg and HBV DNA and enhance the production of IFN-α and antiviral proteins, including p-STAT-1, p-STAT-2, p-JAK1, p-TYK2, OAS1, and Mx, via activation of JAK/STAT signal pathway | Wang et al. (2020c) | |||||
| Influenza A virus | Promotes IFN-γ secretion | Zuo (2008) | |||||
| N-butanol extract | Influenza A virus | The metabolites of extract inhibit the neuraminidase activities | Liu et al. (2012) | ||||
| Extracts | Respiratory syncytial virus | Relieves virus-induced mouse lung lesions and regulates the expression levels of IFN-β and inflammatory cytokines between antiviral and proinflammatory effects via the RIG-I and MDA5 signaling pathways | Xu et al. (2019) | ||||
| Inhibits viral NS1 and L proteins | Zhang (2017) | ||||||
| Influenza A virus | Pretreatment with extract inhibits virus-cell adhesion | Chen et al. (2006) | |||||
| Suppresses the expression of influenza virus nucleoprotein | Xu et al. (2010) | ||||||
| Promotes T, B lymphocytes | Jin (2007) | ||||||
| Inhibits viral entry and impedes viral replication | Fang (2005) | ||||||
| Alleviate the symptoms of virus-infected mice and regulates the immune response by enhancing proliferation and function of T and B cells | Jin (2007) | ||||||
| Rhodiola crenulata (Hook.f. and Thomson) H.Ohba (Hong Jing Tian) | Sweet | Raises qi | Salidroside | Influenza A virus | Relieves lung inflammation in infected mice and reduce the level of inflammatory factors, including IL-1β, IL-6, TNF-α, and C-reactive protein in both serum and lung tissue. Increases the number of CD4 (+) T cells | Lin (2020) | |
| Bitter | Invigorates the blood | Salidroside | Coxsackievirus B3 | Decreases LDH release of infected cardiomyocytes and increase myocardial SOD activity and decreases MDA concentration of CVB3-induced viral myocarditis mice | Wang et al. (2009a) | ||
| Neutral | Alleviate cough | Rhodiola | Coxsackievirus B3 | Decreases LDH release of CVB3-infected viral myocarditis mice | Liu et al. (2002) | ||
| Polysaccharides extract | Coxsackievirus B3 | Inhibits viral replication and protect cardiomyocytes against virus-induced cell apoptosis | Zhang et al. (2009) | ||||
| Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) | Acrid | Transform turbidity with aroma | Patchouli alcohol | Influenza A virus | Inhibits viral infection at the earliest stages of the viral life cycle, including virus attachment and entry | Wei et al. (2013) | |
| Slightly | Check retching | Coxsackievirus B3 | |||||
| Warm | Resolve summerheat | Adenovirus | |||||
| Polyphenolic extracts | Influenza A virus | Inhibits neuraminidase activity | Liu (2016) | ||||
| 4.2 Specific medicinal herbs of JHQGG | |||||||
|---|---|---|---|---|---|---|---|
| Components of medicinal herbs | TCM properties | Key characteristics | Active component | Virus | Pharmacological functions | Referemces | |
| Dryopteris crassirhizoma Nakai (Mian Ma Guan Zhong) | Bitter | Clears internal heat toxin | Dryocrassin ABBA | Influenza A virus | Decreases lung index and virus loads and improves survival rate of H5N1-infected mice. Decreases levels of bronchoalveolar lavage fluid pro-inflammatory cytokines, including IL-6, TNF-α, and IFN-γ, and increases level of anti-inflammatory cytokines, including IL-10 and MCP-1 | Ou et al. (2015) | |
| Stops bleeding | |||||||
| Cold | Kills parasites | Extracts | Influenza A virus | Prevents viral infection and suppresses viral replication | Yang (2010) | ||
| Arctium lappa L. (Niu Bang Zi) | Acrid | Disperses heat in the exterior and clears internal heat toxin | Arctiin | Influenza A virus | Arctigenin could inhibit viral replication and suppress the release of progeny viruses from the host cells. The combination of arctiin and oseltamivir could decrease the virus yields in both bronchoalveolar lavage fluids and lungs than the H1N1-infected mice treated with arctiin or oseltamivir alone | Hayashi et al. (2010) | |
| Bitter | Benefits the throat | Arctigenin | |||||
| Cold | Arctigenin | Japanese encephalitis virus | Anti-inflammatory | Swarup et al. (2008) | |||
| Hydroalcoholic extracts containing arctiin and arctiin | Herpes simplex virus type 1 | Suppress viral replication | Dias et al. (2017) | ||||
| Extracts | Epstein–Barr virus | Suppresses viral replication and decreases viral antigen expression, including capsid antigen and early antigen | Chen and Huang (1994) | ||||
| Anemarrhena asphodeloides Bunge (Zhi Mu) | Bitter | Clears fire and nourishes the Yin of the Lungs, Stomach, and Kidneys | Chinonin | Herpes simplex virus type 2 | Suppresses viral entry and replication | Jiang et al. (2005) | |
| Sweet | Herpes simplex virus type 1 | Inhibits viral replication | Jiang and Xiang (2004) | ||||
| Cold | (—)-(R)-nyasol | Respiratory syncytial virus | Suppresses viral replication more effective than ribavirin | Bae et al. (2007) | |||
| (—)-(R)-4’-O-methylnyasol | |||||||
| Broussonin A | |||||||
| Artemisia annua L. (Qing Hao) | Bitter | Clears all types of yin level heat without injuring the qi, blood, or Yin | Artemisinin | Coxsackievirus B3 | Inhibits viral replication | Ma (2004) | |
| Cold | Cytomegalovirus | Induces early G1 arrest and prevent the progression of cell cycle toward the G1/S checkpoint through reducing the expression of cyclin-dependent kinases 2, 4, and 6 in CMV-infected cells | Roy et al. (2015) | ||||
| Artemisia afra | Human immunodeficiency virus type1 | Inhibits viral replication and release | Lubbe et al. (2012) | ||||
| Polysaccharides extracts | Hepatitis C virus | Acts as an adjuvant in boosting the immune response and promote IFN-γ secretion | Bao et al. (2015) | ||||
| Scutellaria baicalensis Georgi (Huang Qin) | Bitter | Cools heat | Baicalein | Influenza A virus | Suppresses H5N1 replication with antioxidant N-acetyl-l-cysteine combination | Michaelis et al. (2014) | |
| Cold | Dries dampness | Cytomegalovirus | Inhibits viral replication, reduces the levels of virus immediate-early proteins and blocks the nuclear translocation | Evers et al. (2005) | |||
| Stops bleeding | Inhibits viral replication and the expression of vasoactive intestinal peptide in virus-infected human trophoblast cell line | Qiao et al. (2013) | |||||
| Quiets the fetus in pregnancy | Epstein-Barr Virus | Represses Epstein–Barr nuclear antigen1 and Q-promoter activity | Zhang et al. (2018) | ||||
| Human immunodeficiency virus type 1 | Binds to the hydrophobic region of the HIV-1 integrase catalytic core domain | Ahn et al. (2001) | |||||
| Baicalin | Influenza A virus | Protects mice from infection by H1N1 associated with increasing IFN-γ production | Chu et al. (2015) | ||||
| Inhibits virus replication and downregulates the key factors of the RLRs signaling pathway, including RIG-I and NF-κB p65 protein, in H1N1 infected mice | Pang et al. (2018) | ||||||
| Inhibits RNA polymerase activity | Guo et al. (2016) | ||||||
| Interacts with RNA binding domain of Non-structural protein1 | Nayak et al. (2014) | ||||||
| Inhibits viral replication and neuraminidase activity | Sithisarn et al. (2013) | ||||||
| Inhibits TLR7/MyD88 signaling pathway | Wan et al. (2014) | ||||||
| Reduces TNF-α,IL-1 and 5-HT; increases IFN-γ | Li (2019) | ||||||
| Reduces endothelin (ET-1) and ET-1 receptor | Wan (2015) | ||||||
| Chikungunya Virus | Exhibits virucidal activity | Oo et al. (2018) | |||||
| Coxsackie virus B3 | Inhibits viral entry by reducing cellular lipid synthesis | Wang et al. (2020a) | |||||
| Enterovirus 71 | Inhibits viral replication and release by interfering with 3D polymerase transcription and translation | Li et al. (2015) | |||||
| Human immunodeficiency virus type 1 | Inhibits HIV-1 reverse transcriptase activity | Kitamura et al. (1998) | |||||
| Respiratory Syncytial Virus | Increases IFN-1, decreases IL-6, IL-12 | Zhang (2018) | |||||
| Vesicular stomatitis virus | Increases IFN-γ, reduces TNF-α and IL-10 | Orzechowska et al. (2014) | |||||
| Baicalin joint resveratrol | Respiratory Syncytial Virus | Increases serum TNF-α, IL-2, IFN-γ and SIgA in bronchoalveolar lavage fluid | Cheng et al. (2014) | ||||
| Wogonin | Influenza A virus | Suppresses both influenza A and B virus replication in MDCK and A549 cells | Seong et al. (2018) | ||||
| Reduces inflammatory factors | Wu (2011) | ||||||
| 5,7,4′-trihydroxy-8-methoxyflavone | Influenza A virus | Inhibits fusion of virus with endosome/lysosome membrane | Nagai et al. (1995a); Nagai et al. (1995b) | ||||
| 5,7,2′-trihydroxy- and 5,7,2′,3′-tetrahydroxyflavone | Epstein-Barr Virus | Inhibits viral replication and release | Konoshima et al. (1992) | ||||
| Oroxylin A | Influenza A Virus | Inhibits neuraminidase | Jin et al. (2018) | ||||
| Activates the nuclear factor erythroid 2–related factor 2 transcription to increase antioxidant activities | Ji et al. (2015) | ||||||
| Norwogonin, Oroxylin A, mosloflavone | Enterovirus 71 | Inhibits expression of viral capsid proteins | Choi et al. (2016) | ||||
| Artemisinin derivatives | Hepatitis B virus | Reduces viral release | Blazquez et al. (2013) | ||||
| Extract containing baicalein and wogonin | Vesicular stomatitis virus | Inhibits IFN-α and IFN-γ, and stimulates TNF-α and IL (IL-12, IL-10) production | Blach-Olszewska et al. (2008) | ||||
| Flavonoids-enriched extracts | Influenza A virus | Exhibits antiviral activity, including inhibiting viral replication in H1N1-infected MDCK cells, decreasing lung virus titers, reducing hemagglutinin titers and inhibiting neuraminidase activities in lungs of H1N1-infected mice | Zhi et al. (2019) | ||||
| Aqueous extracts | Human immunodeficiency virus type 1 | Inhibits HIV type-1 protease activities | Lam et al. (2000) | ||||
| Fritillaria thunbergii Miq. (Zhe Bei Mu) | Bitter | Cools heat | Extracts | Influenza A virus | Inhibits virus replication in embryonated eggs and reduces H1N1-infected mice mortality rate | Kim et al. (2020) | |
| Cold | Transforms phlegm-heat | ||||||
| Releases constraint | |||||||
| Dissipates nodules, especially in the neck and breast | |||||||
| 4.3 Common medicinal herbs in both prescriptions | ||||||
|---|---|---|---|---|---|---|
| Components of medicinal herbs | TCM properties | Key characteristics | Active component | Virus | Pharmacological functions | References |
| Lonicera japonica Thunb. (Jin Yin Hua) | Sweet | Disperses heat | Chlorogenic acid | Influenza A virus | Suppresses the nucleocapsid protein expression and the release of progeny viruses by inhibiting neuraminidase activity | Ding et al. (2017) |
| Cold | Resolves toxicity | Pheophytin | Hepatitis C virus | Inhibits HCV viral proteins and RNA and exhibits synergistic anti-HCV activity with IFNα-2a | Wang et al. (2009b) | |
| Cools the blood | Honeysuckle-encoded atypical microRNA2911 | Enterovirus 71 | Inhibits EV71 replication by targeting the VP1 gene | Li et al. (2018) | ||
| Stops bleeding | Influenza A virus | Inhibits H1N1, H5N1 and H7N9 viral replication and inhibits H1N1-encoded PB2 and NS1 protein expression. Reduces mouse mortality caused by H5N1 infection | Zhou et al. (2015) | |||
| Polysaccharides extracts | Influenza A virus | Increases serum IFN-γ expression | Zhu (2016) | |||
| Extracts | Respiratory Syncytial Virus | Inhibits virus attachment and replication in Hela cells | Li (2010) | |||
| Dengue virus | Inhibits viral replication and release via the microRNA let-7a targeting viral non-structural protein 1 | Lee et al. (2017) | ||||
| Coxsackie virus B3 | Increases serum SOD activity and decreases MDA concentration of CVB3-induced viral myocarditis mice | Lou (2017) | ||||
| Ephedra sinica Stapf (Ma Huang) | Acrid | Induces sweating | (+)-catechin | Influenza A virus | Suppresses viral replication by inhibiting acidification of endosomes and lysosomes | Mantani et al. (2001) |
| Slightly bitter | Calms wheezing | L-methylephedrin, L-ephedrine, D-pseudo- ephedrine | Influenza A virus | Increases IFN-β and decreases TNF-α level by regulating TLRs and RIG-1 pathways | Wei et al. (2019) | |
| Warm | Promotes urination | Water Extract | Respiratory syncytial virus | Inhibits viral absorption and penetration | Zhu and Li (2012) | |
| Forsythia suspensa (Thunb.) Vahl (Lian Qiao) | Bitter | Cools and vents heat, particularly in the Heart and upper burner | Forsythoside A | Influenza A virus | Inhibits virus spread by reducing influenza viral M1 protein | Law et al. (2017) |
| Slightly acrid | Resolves toxicity | Reduces TLR7, MyD88 and NF-κB p65 protein | Deng et al. (2016) | |||
| Slightly cold | Disperses clumps | Phillyrin | Influenza A virus | Decreases IL-6 levels, and reduces the expression of hemagglutinin in mice infected with influenza A virus | Qu et al. (2016) | |
| Mentha canadensis L. (Bo He) | Acrid | Facilitates the dispersal of upper burner wind-heat | Essential oil extract, piperitenone oxide | Herpes simplex virus type 1 | Inhibits viral replication | Civitelli et al. (2014) |
| Aromatic | Cools and clears the eyes and head | |||||
| Cooling | Soothers the throat | |||||
| Facilitates the flow of Liver qi and expels turbid filth | ||||||
| Glycyrrhiza glabra L. (Gan Cao) | Sweet | Tonifies the Spleen qi | Glycyrrhizin | Influenza A virus | Reduces endocytosis activity and virus uptake | Wolkerstorfer et al. (2009) |
| Neutral | Moistens the Lungs | Inhibits influenza virus polymerase activity | Moisy et al. (2012) | |||
| Moderates urgency and toxicity | Glycyrrhizic acid | Enterovirus 71 | Inhibits viral replication | Wang et al. (2013) | ||
| Drains fire | Chalcones | Influenza A virus | Inhibits neuraminidase activity | Dao et al. (2011) | ||
| Triterpene glycyrrhizic acid | Herpes simplex virus type 1 | Induces autophagy activator Beclin 1 to establish a resistance state to viral replication | Laconi et al. (2014) | |||
| 18β-glycyrrhetinic acid | Ebola virus | Binds to nucleoprotein | Fu et al. (2016) | |||
| A glycyrrhizin-containing preparation | Hepatitis C virus | Protects mitochondria against oxidative stress | Korenaga et al. (2011) | |||
| Water extracts | Respiratory syncytial virus | Induces IFN-β secretion | Yeh et al. (2013) | |||
| Ethanol extracts | Influenza A virus | Suppresses RANTES secretion | Ko et al. (2006) | |||
In terms of COVID-19, the ACE-2 has been identified as the most important receptor for SARS-CoV-2 viral entry, which constitutes the initial step of infection (Walls et al., 2020). Through informatic analysis, the Rheum palmatum L (Da Huang) in LHQWC was found to be able to suppress viral infection by directly blocking interactions between the spike protein and ACE2. In addition, in the SARS-CoV, MERS-CoV and other coronaviruses, the 3CL (3C-like) protease is one of the crucial enzymes that mediates viral replication and has been recognized as a potential therapeutic target (Pillaiyar et al., 2016; Galasiti Kankanamalage et al., 2018). These predictive evaluations showed that Scutellaria baicalensis Georgi (Huang Qin), Anemarrhena asphodeloides Bunge (Zhi Mu) and Arctium lappa L (Niu Bang Zi) in JHQGG, as well as Rheum palmatum L (Da Huang) and Houttuynia cordata Thunb (Yu Xing Cao) in LHQWC can inhibit viral transcription and replication, especially that the Rheum palmatum L (Da Huang) in LHQWC was shown as a potential inhibitor of 3CL protease, suggesting underlying mechanisms of both LHQWC and JHQGG in the treatment of COVID-19.
Since LHQWC and JHQGG are both commonly used for the treatment of influenza in China, we additionally analyzed their possible roles in the inhibition of influenza viral invasion. Hemagglutinin (HA) on the surface of influenza virus is a tri-polymer, which promotes virus binding and entering into host cells. In contrast to HA, the neuraminidase (NA) of influenza viruses involves detachment and release of mature viruses from host cells (Gamblin and Skehel, 2010; Gaymard et al., 2016). Components of Scutellaria baicalensis Georgi (Huang Qin) of JHQGG have been shown to inhibit the whole life cycle of influenza viruses, such as inhibiting HA and NA, and suppressing replicons. Meanwhile, Isatis tinctoria L (Ban Lan Gen) and Rheum palmatum L (Da Huang) of LHQWC have also been reported to reduce the internalization and replication of influenza viruses. The shared herbs, such as Ephedra sinica Stapf (Ma Huang), Lonicera japonica Thunb (Jin Yin Hua), Forsythia suspensa (Thunb.) Vahl (Lian Qiao) and Glycyrrhiza glabra L (Gan Cao) in both LHQWC and JHQGG were experimentally proved as inhibitors of influenza virus life cycle (Table 1; Table 1).
Discussion
In clinical practices of TCM, medicinal herbs are generally applied in the form of decoctions, which contain mixtures of a variety of herbs with different pharmacological functions. Instead of directly inactivating pathogens, therapeutic effects of TCM decoctions are achieved mainly through balancing host anti-viral responses and pathogenic factors. During COVID-19 epidemics, synergistic therapy of LHQWC with clinically approved reproposing antivirals, such as oseltamivir, umifenovir, ribavirin, lopinavir, peramivir, penciclovir or ganciclovir, has shown its advantages in improving associated symptoms and reducing the course of hospitalization and disease progression in several reported trials (Liu M. et al., 2020; Yu, 2020a; Yu, 2020b; Cheng, 2020; Hu et al., 2020; Li et al., 2020; Lv and Wang, 2020; Xiao et al., 2020; Chen, 2021; Liu et al., 2021). Similarly, combined anti-viral treatment with JHQGG in mild or moderate COVID-19 was beneficial in relieving clinical symptoms and reducing risks of severe COVID-19 (Liu Z. et al., 2020; Duan, 2020; Duan, 2020). These studies provide clinical evidence that combined treatment with either LHQWC or JHQGG is superior to conventional monotherapy of antivirals.
The primary conclusion of our study that both LHQWC and JHQGG are efficient for a large range of viral diseases has supported that TCM formulae can be potentially an alternative therapy for emerging viral diseases, especially when specific drugs and vaccines have not been fully developed and applied. However, when it comes to appropriate or precisive clinical applications of LHQWC and JHQGG, differences of their associated pharmacological actions turn out to be an essential point to be addressed. When comparing the anti-viral targets of LHQWC and JHQGG, both CPMs have been documented effective in interfering with viral components, with Isatis tinctoria L (Ban Lan Gen) and Rheum palmatum L (Da Huang) in LHQWC being the predominate viral inhibitors, followed by Lonicera japonica Thunb (Jin Yin Hua) and Houttuynia cordata Thunb (Yu Xing Cao). While in JHQGG, the Scutellaria baicalensis Georgi (Huang Qin) and subsequently Lonicera japonica Thunb (Jin Yin Hua) are the most important virucidal herbs. Typically, Scutellaria baicalensis Georgi (Huang Qin) of JHQGG have been highly nominated among all analyzed herbs contributing to suppression of the whole viral life cycle. Intriguingly, a direct virucidal activity was observed mostly in components from Scutellaria baicalensis Georgi (Huang Qin) and Anemarrhena asphodeloides Bunge (Zhi Mu) of JHQGG, though shared herbs, Lonicera japonica Thunb (Jin Yin Hua) and Glycyrrhiza glabra L (Gan Cao) were also involved. This set of data indicate that from the angle of viral life cycle, JHQGG may overweight LHQWC due to Scutellaria baicalensis Georgi (Huang Qin), and will be appropriate for patients with high fever, sore throat and cough. On the other hand, owning to existence of Rhodiola crenulata (Hook.f. and Thomson) H. Ohba (Hong Jing Tian), LHQWC may have more essential roles in the balancing of host immunity, suggesting that LHQWC could be more suitable for patients with non-efficient anti-viral immune responses.
There are some possible limitations in this study. Firstly, based on five databases, we finally included relatively more articles associated with LHQWC compared with those of JHQGG; therefore, bias could be unintendedly introduced to conclusions supporting superiority of LHQWC. Secondly, a certain number of included studies focus on Scutellaria baicalensis Georgi (Huang Qin), Isatis tinctoria L (Ban Lan Gen) and Rheum palmatum L (Da Huang); therefore, this may lead to biases that only these herbs are important as antivirals. Thirdly, the quality of articles included in this study is variable, and the judgment for potential pharmacological actions may to some degree rely on the knowledge of authors.
COVID-19 initiates with mild or moderate symptoms in most cases, and the strategy to reduce risks in evolving into severe or critical COVID-19 is highly desired. Through literature mining, we provide general evidence that both LHQWC and JHQGG are effective for mild to moderate COVID-19 patients and potentially being able to prevent the progress of COVID-19 into severe or critical conditions. As discussed above, TCM therapy fits well with the principle of HDT, and anti-viral TCM formulae generally show a broad spectrum of anti-viral properties through balancing between viral activities and host immune reactions. This has gained TCM a key advantage over target-specific anti-viral medications. Since LHQWC and JHQGG are both CPMs with clear safety information, it is imperative that application of LHQWC and JHQGG can be contextualized to worldwide combat against the emerging or re-emerging of human pandemics.
Acknowledgments
Authors thank Zhenji LI, World Federation of Chinese Medicine Societies for his supports and valuable input.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Author Contributions
NL, RX and JL initiated and supervised this study. NL, RX, MS, BP, and AL performed data analysis and wrote this manuscript. PS assisted in organizing and analyzing data, and ZL contributed to editing.
Funding
This research was funded by a grant from the Key Projects for International Cooperation on Science, Technology and Innovation (2020YFE0205100), and Fundamental Scientific Research of Central Public Welfare Foundation from China Academy of China Medical Sciences (YZ-202012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahn H., Lee S. Y., Kim J. W., Son W. S., Shin C. G., Lee B. J. (2001). Binding Aspects of Baicalein to HIV-1 Integrase. Mol. Cells 12 (1), 127–130. [PubMed] [Google Scholar]
- Bae G., Yu J.-R., Lee J., Chang J., Seo E.-K. (2007). Identification of Nyasol and Structurally Related Compounds as the Active Principles fromAnemarrhena Asphodeloides against Respiratory Syncytial Virus (RSV). Chem. Biodivers. 4 (9), 2231–2235. 10.1002/cbdv.200790181 [DOI] [PubMed] [Google Scholar]
- Bao L. D., Ren X. H., Ma R. L., Wang Y., Yuan H. W., Lv H. J. (2015). Efficacy of Artemisia Annua Polysaccharides as an Adjuvant to Hepatitis C Vaccination. Genet. Mol. Res. 14 (2), 4957–4965. 10.4238/2015.may.11.29 [DOI] [PubMed] [Google Scholar]
- Biswas N. K., Majumder P. P. (2020). Analysis of RNA Sequences of 3636 SARS-CoV-2 Collected from 55 Countries Reveals Selective Sweep of One Virus Type. Indian J. Med. Res. 151, 450-458. 10.4103/ijmr.IJMR_1125_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blach-Olszewska Z., Jatczak B., Rak A., Lorenc M., Gulanowski B., Drobna A., et al. (2008). Production of Cytokines and Stimulation of Resistance to Viral Infection in Human Leukocytes by Scutellaria Baicalensis Flavones. J. Interferon Cytokine Res. 28 (9), 571–581. 10.1089/jir.2008.0125 [DOI] [PubMed] [Google Scholar]
- Blazquez A. G., Fernandez-Dolon M., Sanchez-Vicente L., Maestre A. D., Gomez-San Miguel A. B., Alvarez M., et al. (2013). Novel Artemisinin Derivatives with Potential Usefulness against Liver/colon Cancer and Viral Hepatitis. Bioorg. Med. Chem. 21 (14), 4432–4441. 10.1016/j.bmc.2013.04.059 [DOI] [PubMed] [Google Scholar]
- Cai Z., Luo Y. (2014). The Protective Effect and Mechanism of Emodin on Experimental Viral Myocarditis in Mice. Guangdong Medicial J. 35 (9), 1326–1329. 10.13820/j.cnki.gdyx.2014.09.012 [DOI] [Google Scholar]
- Chen C., Li X., Liu Y., Chen S. (2021). Clinical Study of Lianhua Qingwen Capsule in the Treatment of Corona Virus Disease 2019. Res. Integrated Traditional Chin. West. Med. 13 (1), 1–4. 10.3969/j.issn.1674-4616.2021.01.001 [DOI] [Google Scholar]
- Chen M., Li H., Lu X., Ling L., Weng H., Sun W., et al. (2019). Houttuynia Cordata Polysaccharide Alleviated Intestinal Injury and Modulated Intestinal Microbiota in H1N1 Virus Infected Mice. Chin. J. Nat. Medicines 17 (3), 187–197. 10.1016/s1875-5364(19)30021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Huang D. (1994). The Inhibitory Effect of Burdock on the Expression of Epstein-Barr Virus Antigen. Chin. J. Exp. Clin. Virol. 8 (4), 323–326. [Google Scholar]
- Chen Y., Luo Q., Li S., Li C., Liao S., Yang X., et al. (2020). Antiviral Activity against Porcine Epidemic Diarrhea Virus of Pogostemon Cablin Polysaccharide. J. Ethnopharmacology 259, 113009. 10.1016/j.jep.2020.113009 [DOI] [PubMed] [Google Scholar]
- Chen Z., Wu L. W., Liu S. T., Cai C. P., Rao P. F., Ke L. J. (2006). Mechanism Study of Anti-influenza Effects of Radix Isatidis Water Extract by Red Blood Cells Capillary Electrophoresis. Zhongguo Zhong Yao Za Zhi 31 (20), 1715–1719. 10.3321/j.issn:1001-5302.2006.20.019 [DOI] [PubMed] [Google Scholar]
- Cheng D., Wang W., Li Y., Wu X., Zhou B., Song Q. (2020). Analysis of Curative Effect of 51 Patients with Novel Coronavirus Pneumonia Treated with Chinese Medicine Lianhua Qingwen:a Multicentre Retrospective Study. Tianjin Traditional Chin. Med. 37 (5), 509–516. 10.11656/j.issn.1672-1519.2020.05.06 [DOI] [Google Scholar]
- Cheng K., Wu Z., Gao B., Xu J. (2014). Analysis of Influence of Baicalin Joint Resveratrol Retention Enema on the TNF-α, SIgA, IL-2, IFN-γ of Rats with Respiratory Syncytial Virus Infection. Cell Biochem Biophys. 70 (2), 1305–1309. 10.1007/s12013-014-0055-9 [DOI] [PubMed] [Google Scholar]
- Choi H. J., Song H.-H., Lee J.-S., Ko H.-J., Song J.-H. (2016). Inhibitory Effects of Norwogonin, Oroxylin A, and Mosloflavone on Enterovirus 71. Biomolecules Ther. (Seoul) 24 (5), 552–558. 10.4062/biomolther.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M., Xu L., Zhang M. B., Chu Z. Y., Wang Y. D. (2015). Role of Baicalin in Anti-influenza Virus A as a Potent Inducer of IFN-Gamma. Biomed. Res. Int. 2015, 263630. 10.1155/2015/263630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitelli L., Panella S., Marcocci M. E., De Petris A., Garzoli S., Pepi F., et al. (2014). In vitro inhibition of Herpes Simplex Virus Type 1 Replication by Mentha Suaveolens Essential Oil and its Main Component Piperitenone Oxide. Phytomedicine 21 (6), 857–865. 10.1016/j.phymed.2014.01.013 [DOI] [PubMed] [Google Scholar]
- Dao T. T., Nguyen P. H., Lee H. S., Kim E., Park J., Lim S. I., et al. (2011). Chalcones as Novel Influenza A (H1N1) Neuraminidase Inhibitors from Glycyrrhiza Inflata. Bioorg. Med. Chem. Lett. 21 (1), 294–298. 10.1016/j.bmcl.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Deng L., Pang P., Zheng K., Nie J., Xu H., Wu S., et al. (2016). Forsythoside A Controls Influenza A Virus Infection and Improves the Prognosis by Inhibiting Virus Replication in Mice. Molecules 21 (5). 10.3390/molecules21050524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias M. M., Zuza O., Riani L. R., de Faria Pinto P., Pinto P. L. S., Silva M. P., et al. (2017). In vitro schistosomicidal and Antiviral Activities of Arctium Lappa L. (Asteraceae) against Schistosoma Mansoni and Herpes Simplex Virus-1. Biomed. Pharmacother. 94, 489–498. 10.1016/j.biopha.2017.07.116 [DOI] [PubMed] [Google Scholar]
- Ding Y., Cao Z., Cao L., Ding G., Wang Z., Xiao W. (2017). Antiviral Activity of Chlorogenic Acid against Influenza A (H1N1/H3N2) Virus and its Inhibition of Neuraminidase. Sci. Rep. 7, 45723. 10.1038/srep45723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Xia W., Zheng Q., Sun G., LI Z., Li Q., et al. (2020). Clinical Observation on Jinhua Qinggan Granule Combined with Conventional Western Medicine Therapy in Treating Mild Cases of Coronavirus Disease 2019. J. traditional Chin. Med. 61 (17), 1473–1477. 10.13288/j.11-2166/r.2020.17.001 [DOI] [Google Scholar]
- Esposito F., Carli I., Del Vecchio C., Xu L., Corona A., Grandi N., et al. (2016). Sennoside A, Derived from the Traditional Chinese Medicine Plant Rheum L., Is a New Dual HIV-1 Inhibitor Effective on HIV-1 Replication. Phytomedicine 23 (12), 1383–1391. 10.1016/j.phymed.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Evers D. L., Chao C.-F., Wang X., Zhang Z., Huong S.-M., Huang E.-S. (2005). Human Cytomegalovirus-Inhibitory Flavonoids: Studies on Antiviral Activity and Mechanism of Action. Antiviral Res. 68 (3), 124–134. 10.1016/j.antiviral.2005.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Tang J., Yang Z., Hu Y., Liu Y., Wang W. (2005). Effect of Radix Isatidis against Herpes Simplex Virus Type Ⅰ In Vitro. Chin. Traditional Herbal Drugs 36 (2), 242–244. 10.3321/j.issn:0253-2670.2005.02.034 [DOI] [Google Scholar]
- Fu X., Wang Z., Li L., Dong S., Li Z., Jiang Z., et al. (2016). Novel Chemical Ligands to Ebola Virus and Marburg Virus Nucleoproteins Identified by Combining Affinity Mass Spectrometry and Metabolomics Approaches. Sci. Rep. 6, 29680. 10.1038/srep29680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasiti Kankanamalage A. C., Kim Y., Damalanka V. C., Rathnayake A. D., Fehr A. R., Mehzabeen N., et al. (2018). Structure-guided Design of Potent and Permeable Inhibitors of MERS Coronavirus 3CL Protease that Utilize a Piperidine Moiety as a Novel Design Element. Eur. J. Med. Chem. 150, 334–346. 10.1016/j.ejmech.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin S. J., Skehel J. J. (2010). Influenza Hemagglutinin and Neuraminidase Membrane Glycoproteins. J. Biol. Chem. 285 (37), 28403–28409. 10.1074/jbc.r110.129809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard A., Le Briand N., Frobert E., Lina B., Escuret V. (2016). Functional Balance between Neuraminidase and Haemagglutinin in Influenza Viruses. Clin. Microbiol. Infect. 22 (12), 975–983. 10.1016/j.cmi.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Guan W.‐j., Ni Z. Y., Hu Y., Liang W. H., Ou C. Q., He J., et al. (2020). Clinical Characteristics of 2019 Novel Coronavirus Infection in China . N. Engl. J. Med. 382 (18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Bao L., Cui X. (2016). Effects of Baicalin on Activity of Influenza A Virus RNA Polymerase by Silencing Host Factors PACT. Chin. J. Pharmacovigilance 13 (3), 129–131. 10.19803/j.1672-8629.2016.03.001 [DOI] [Google Scholar]
- Hayashi K., Narutaki K., Nagaoka Y., Hayashi T., Uesato S. (2010). Therapeutic Effect of Arctiin and Arctigenin in Immunocompetent and Immunocompromised Mice Infected with Influenza A Virus. Biol. Pharm. Bull. 33 (7), 1199–1205. 10.1248/bpb.33.1199 [DOI] [PubMed] [Google Scholar]
- He F., Liu Q., Wei F., Liu Y., Xiong H., Zhou X., et al. (2013). Anti-viral Activity of Rhubarb Extract and Emodin in Rotavirus-Infected Cells. Chin. J. Viral Dis. 3 (2), 112–116. 10.16505/j.2095-0136.2013.02.005 [DOI] [Google Scholar]
- He L., Fan F., Hou X., Wu H., Wang J., Xu H., et al. (2017). 4(3H)-Quinazolone Regulates Innate Immune Signaling upon Respiratory Syncytial Virus Infection by Moderately Inhibiting the RIG-1 Pathway in RAW264.7 Cell. Int. Immunopharmacology 52, 245–252. 10.1016/j.intimp.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Ho T., Wu S., Chen J., Li C., Hsiang C. (2007). Emodin Blocks the SARS Coronavirus Spike Protein and Angiotensin-Converting Enzyme 2 Interaction. Antiviral Res. 74 (2), 92–101. 10.1016/j.antiviral.2006.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Guan W. J., Bi Y., Zhang W., Li L., Zhang B., et al. (2020). Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 85, 153242. 10.1016/j.phymed.2020.153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung P.-Y., Ho B.-C., Lee S.-Y., Chang S.-Y., Kao C.-L., Lee S.-S., et al. (2015). Houttuynia Cordata Targets the Beginning Stage of Herpes Simplex Virus Infection. PLoS One 10 (2), e0115475. 10.1371/journal.pone.0115475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcovich A., Siemieniuk R., Bartoszko J., Ge L., Zeraatkar D., Kum E., et al. (2020). Adverse Effects of Remdesivir, Hydroxychloroquine, and Lopinavir/Ritonavir When Used for COVID-19: Systematic Review and Meta-Analysis of Randomized Trials. Preprint at https://www.medrxiv.org/content/10.1101/2020.11.16.20232876v1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., Li R., Wang Q., Miao W.-j., Li Z.-w., Si L.-l., et al. (2015). Anti-H1N1 Virus, Cytotoxic and Nrf2 Activation Activities of Chemical Constituents from Scutellaria Baicalensis. J. Ethnopharmacology 176, 475–484. 10.1016/j.jep.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Jia W., Mao S., Zhang P., Yan G., Jin J., Liu Y. (2018). Study on Antiviral Effect of Lonicera Japonica Thumb Polysaccharide In Vivo. J. Liaoning Univ. Traditional Chin. Med. 20 (6), 25–27. 10.13194/j.issn.1673-842x.2018.06.007 [DOI] [Google Scholar]
- Jia Y., Xu R., Hu Y., Zhu T., Ma T., Wu H., et al. (2016). Anti-NDV Activity of Baicalin from a Traditional Chinese Medicine In Vitro. J. Vet. Med. Sci. 78 (5), 819–824. 10.1292/jvms.15-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Li S., Li M., Xiang J. (2005). Anti-viral Effects of Chinonin against HSV-Ⅱ In Vitro. Acta Medicinae Universitatis Scientiae Et Technologiae Huazhong 34 (3), 304–307. [Google Scholar]
- Jiang J., Xiang J. (2004). Study on Activity of Chinonin against HSV-Ⅰ In Vitro. China Pharmacist 7 (9), 666–670. 10.3969/j.issn.1008-049X.2004.09.004 [DOI] [Google Scholar]
- Jiang N., Liao W., Kuang X. (2014). Effects of Emodin on IL-23/IL-17 Inflammatory axis, Th17 Cells and Viral Replication in Mice with Viral Myocarditis. Nan Fang Yi Ke Da Xue Xue Bao 34 (3), 373–378. [PubMed] [Google Scholar]
- Jin J., Chen S., Wang D., Chen Y., Wang Y., Guo M., et al. (2018). Oroxylin A Suppresses Influenza A Virus Replication Correlating with Neuraminidase Inhibition and Induction of IFNs. Biomed. Pharmacother. 97, 385–394. 10.1016/j.biopha.2017.10.140 [DOI] [PubMed] [Google Scholar]
- Jin M., Ren D., Meng F., Li X. (2007). The Effects of Radix Isatidis on Immunological Function and Influenza Virus (FM1) in Kunming Mice. Lishizhen Med. Materia Med. Res. 18 (2), 394–396. 10.3969/j.issn.1008-0805.2007.02.073 [DOI] [Google Scholar]
- Kaufmann S. H. E., Dorhoi A., Hotchkiss R. S., Bartenschlager R. (2018). Host-directed Therapies for Bacterial and Viral Infections. Nat. Rev. Drug Discov. 17 (1), 35–56. 10.1038/nrd.2017.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khider L., Gendron N., Goudot G., Chocron R., Hauw-Berlemont C., Cheng C., et al. (2020). Curative Anticoagulation Prevents Endothelial Lesion in COVID-19 Patients. J. Thromb. Haemost. 18, 2391-2399. 10.1111/jth.14968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Nguyen D.-V., Heo Y., Park K. H., Paik H.-D., Kim Y. B. (2020). Antiviral Activity of Fritillaria Thunbergii Extract against Human Influenza Virus H1N1 (PR8) In Vitro, in Ovo and In Vivo . J. Microbiol. Biotechnol. 30 (2), 172–177. 10.4014/jmb.1908.08001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Honda M., Yoshizaki H., Yamamoto S., Nakane H., Fukushima M., et al. (1998). Baicalin, an Inhibitor of HIV-1 Production In Vitro. Antiviral Res. 37 (2), 131–140. 10.1016/s0166-3542(97)00069-7 [DOI] [PubMed] [Google Scholar]
- Ko H.-C., Wei B.-L., Chiou W.-F. (2006). The Effect of Medicinal Plants Used in Chinese Folk Medicine on RANTES Secretion by Virus-Infected Human Epithelial Cells. J. Ethnopharmacology 107 (2), 205–210. 10.1016/j.jep.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Konoshima T., Kokumai M., Kozuka M., Iinuma M., Mizuno M., Tanaka T., et al. (1992). Studies on Inhibitors of Skin Tumor Promotion. XI. Inhibitory Effects of Flavonoides from Scutellaria Baicalensis on Epstein-Barr Virus Activation and Their Anti-tumor-promoting Activities. Chem. Pharm. Bull (Tokyo) 40 (2), 531–533. 10.1248/cpb.40.531 [DOI] [PubMed] [Google Scholar]
- Korenaga M., Hidaka I., Nishina S., Sakai A., Shinozaki A., Gondo T., et al. (2011). A Glycyrrhizin-Containing Preparation Reduces Hepatic Steatosis Induced by Hepatitis C Virus Protein and Iron in Mice. Liver Int. 31 (4), 552–560. 10.1111/j.1478-3231.2011.02469.x [DOI] [PubMed] [Google Scholar]
- Laconi S., Madeddu M. A., Pompei R. (2014). Autophagy Activation and Antiviral Activity by a Licorice Triterpene. Phytother. Res. 28 (12), 1890–1892. 10.1002/ptr.5189 [DOI] [PubMed] [Google Scholar]
- Lam T. L., Lam M. L., Au T. K. K., Ip D. T. M., Ng T. B., Fong W. P., et al. (2000). A Comparison of Human Immunodeficiency Virus Type-1 Protease Inhibition Activities by the Aqueous and Methanol Extracts of Chinese Medicinal Herbs. Life Sci. 67 (23), 2889–2896. 10.1016/s0024-3205(00)00864-x [DOI] [PubMed] [Google Scholar]
- Lau K.-M., Lee K.-M., Koon C.-M., Cheung C. S.-F., Lau C.-P., Ho H.-M., et al. (2008). Immunomodulatory and Anti-SARS Activities of Houttuynia Cordata. J. Ethnopharmacology 118 (1), 79–85. 10.1016/j.jep.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. H.-Y., Yang C. L.-H., Lau A. S.-Y., Chan G. C.-F. (2017). Antiviral Effect of Forsythoside A from Forsythia Suspensa (Thunb.) Vahl Fruit against Influenza A Virus through Reduction of Viral M1 Protein. J. Ethnopharmacology 209, 236–247. 10.1016/j.jep.2017.07.015 [DOI] [PubMed] [Google Scholar]
- Lee Y.-R., Yeh S.-F., Ruan X.-M., Zhang H., Hsu S.-D., Huang H.-D., et al. (2017). Honeysuckle Aqueous Extract and Induced Let-7a Suppress Dengue Virus Type 2 Replication and Pathogenesis. J. Ethnopharmacology 198, 109–121. 10.1016/j.jep.2016.12.049 [DOI] [PubMed] [Google Scholar]
- Li H., Wu J., Zhang Z., Ma Y., Liao F., Zhang Y., et al. (2011). Forsythoside a Inhibits the Avian Infectious Bronchitis Virus in Cell Culture. Phytother. Res. 25 (3), 338–342. 10.1002/ptr.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., et al. (2017). Bioinformatics Analysis on Effect of Lonicerae Japonicae Flos and Forsythiae Fructus on Immune Pathway of H1N1 Influenza A. Chin. J. Exp. Traditional Med. Formulae 23 (10), 201–204. [Google Scholar]
- Li S.-W., Yang T.-C., Lai C.-C., Huang S.-H., Liao J.-M., Wan L., et al. (2014). Antiviral Activity of Aloe-Emodin against Influenza A Virus via Galectin-3 Up-Regulation. Eur. J. Pharmacol. 738, 125–132. 10.1016/j.ejphar.2014.05.028 [DOI] [PubMed] [Google Scholar]
- Li T., Liu L., Wu H., Chen S., Zhu Q., Gao H., et al. (2017b). Anti-herpes Simplex Virus Type 1 Activity of Houttuynoid A, a Flavonoid from Houttuynia Cordata Thunb. Antiviral Res. 144, 273–280. 10.1016/j.antiviral.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Li W., et al. (2019). Effect of Baicalin on Blood Index in Mice Infected H6 N6 Avian Influenza Virus. Chin. J. Vet. Drug 53 (10), 61–70. [Google Scholar]
- Li X., Huang Y., Sun M., Ji H., Dou H., Hu J., et al. (2018). Honeysuckle-encoded microRNA2911 Inhibits Enterovirus 71 Replication via Targeting VP1 Gene. Antiviral Res. 152, 117–123. 10.1016/j.antiviral.2018.02.015 [DOI] [PubMed] [Google Scholar]
- Li X., Liu Y., Wu T., Jin Y., Cheng J., Wan C., et al. (2015). The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro . Viruses 7 (8), 4756–4771. 10.3390/v7082841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang Y., Liu L., Yang X., Zhao X., Li Y., et al. (2020). Effect of Combination Antiviral Therapy on Hematological Profiles in 151 Adults Hospitalized with Severe Coronavirus Disease 2019. Pharmacol. Res. 160, 105036. 10.1016/j.phrs.2020.105036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Li L., Zhou H., Zeng L., Chen T., Chen Q., et al. (2017a). Radix Isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro . Molecules 22 (1). 10.3390/molecules22010116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Huang Y., Pan X., Hao Y., Chen X., Jiang H., et al. (2020). Erucic Acid from Isatis Indigotica Fort. Suppresses Influenza A Virus Replication and Inflammation In Vitro and In Vivo through Modulation of NF-Κb and P38 MAPK Pathway. J. Pharm. Anal. 10 (2), 130–146. 10.1016/j.jpha.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-Y., Liu Y.-C., Jheng J.-R., Tsai H.-P., Jan J.-T., Wong W.-R., et al. (2009). Anti-enterovirus 71 Activity Screening of Chinese Herbs with Anti-infection and Inflammation Activities. Am. J. Chin. Med. 37 (1), 143–158. 10.1142/s0192415x09006734 [DOI] [PubMed] [Google Scholar]
- Lin W., et al. (2020). Influence of Salidroside on Serum and Lung Tissue Inflammatory Factors and Immunological Indexes of Mice Infected with Influenza Virus. Chin. J. Nosocomiology 30 (2), 292–296. [Google Scholar]
- Ling L.-j., Lu Y., Zhang Y.-y., Zhu H.-y., Tu P., Li H., et al. (2020). Flavonoids from Houttuynia Cordata Attenuate H1N1-Induced Acute Lung Injury in Mice via Inhibition of Influenza Virus and Toll-like Receptor Signalling. Phytomedicine 67, 153150. 10.1016/j.phymed.2019.153150 [DOI] [PubMed] [Google Scholar]
- Liu F., et al. (2016a). Polyphenolic Glycosides Isolated from Pogostemon Cablin (Blanco) Benth. As Novel Influenza Neuraminidase Inhibitors. Chem. Cent. J. 10, 51. 10.1186/s13065-016-0192-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Shi F., Tu P., Chen C., Zhang M., Li X., et al. (2021). Arbidol Combined with the Chinese Medicine Lianhuaqingwen Capsule versus Arbidol Alone in the Treatment of COVID-19. Medicine (Baltimore) 100 (4), e24475. 10.1097/md.0000000000024475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., et al. (2020). Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a Systematic Review and Meta-Analysis. Pharmacol. Res. 158, 104896. 10.1016/j.phrs.2020.104896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yan J., Xing J., Song F., Liu Z., Liu S. (2012). Characterization of Compounds and Potential Neuraminidase Inhibitors from the N-Butanol Extract of Compound Indigowoad Root Granule Using Ultrafiltration and Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 59, 96–101. 10.1016/j.jpba.2011.10.015 [DOI] [PubMed] [Google Scholar]
- Liu X., Yang Y., Zhou T., Zhang J., Yang X., Chen H. (2002). The Effects of Astragalus Membranaceus, Rhodilolea and FTY720 on Murine Virus Mvocarditis Model Induced by Coxsackievirus B3. Mol. Cardiol. China 2 (3), 17–22. [Google Scholar]
- Liu Y., et al. (2016b). Antiviral Effects of Three Chinese Herbal Medicine and Their Polysaccharides on Porcine Reproductive and Respiratory Syndrome Virus (PRRSV)In Vitro. China Anim. Husbandry Vet. Med. 43 (10), 2730–2735. [Google Scholar]
- Liu Z., Li X., Gou C., Li L., Luo X., Zhang C., et al. (2020b). Effect of Jinhua Qinggan Granules on Novel Coronavirus Pneumonia in Patients. J. Tradit Chin. Med. 40 (3), 467–472. 10.19852/j.cnki.jtcm.2020.03.016 [DOI] [PubMed] [Google Scholar]
- Liu Z., Ma N., Zhong Y., Yang Z.-q. (2015). Antiviral Effect of Emodin from Rheum Palmatum against Coxsakievirus B5 and Human Respiratory Syncytial Virus In Vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 35 (6), 916–922. 10.1007/s11596-015-1528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X., Hu J., Ge D., Lu W. (2017). Protective Effect of Honeysuckle on Viral Myocarditis in Mice and its Mechanism. J. Traditional Chin. Med. 45 (1), 37–41. 10.3969/j.issn.1002-2392.2017.01.010 [DOI] [Google Scholar]
- Lubbe A., Seibert I., Klimkait T., van der Kooy F. (2012). Ethnopharmacology in Overdrive: the Remarkable Anti-HIV Activity of Artemisia Annua. J. Ethnopharmacology 141 (3), 854–859. 10.1016/j.jep.2012.03.024 [DOI] [PubMed] [Google Scholar]
- Luo W., Su X., Gong S. (2009). Anti-SARS Coronavirus 3C-like Protease Effects of Rheum Palmatum L. Extracts. Biosci. Trends 3 (4), 124–126. https://www.biosciencetrends.com/article/3/4/124 [PubMed] [Google Scholar]
- Luo Z., Liu L. F., Wang X. H., Li W., Jie C., Chen H., et al. (2019). Epigoitrin, an Alkaloid from Isatis Indigotica, Reduces H1N1 Infection in Stress-Induced Susceptible Model In Vivo and In Vitro. Front. Pharmacol. 10, 78. 10.3389/fphar.2019.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R., Wang W. L. X. (2020). Clinical Observation on Lianhua Qingwen Granules Combined with Western Medicine Conventional Therapy in the Treatment of 63 Suspected Cases of Coronavirus Disease 2019. J. Traditional Chin. Med. 6 (18), 655–659. [Google Scholar]
- Ma P., et al. (2004). Study on Anti-Coxsackie Virus B3 Effect of Artemisinin. Chin. J. Endemiology 23 (5), 403–405. [Google Scholar]
- Mantani N., Imanishi N., Kawamata H., Terasawa K., Ochiai H. (2001). Inhibitory Effect of (+)-catechin on the Growth of Influenza A/PR/8 Virus in MDCK Cells. Planta Med. 67 (3), 240–243. 10.1055/s-2001-12009 [DOI] [PubMed] [Google Scholar]
- Meo S. A., Alhowikan A. M., Al-Khlaiwi T., Meo I. M., Halepoto D. M., Iqbal M., et al. (2020). Novel Coronavirus 2019-nCoV: Prevalence, Biological and Clinical Characteristics Comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 24 (4), 2012–2019. 10.26355/eurrev_202002_20379 [DOI] [PubMed] [Google Scholar]
- Michaelis M., Sithisarn P., Cinatl Jr J., Jr. (2014). Effects of Flavonoid-Induced Oxidative Stress on Anti-h5n1 Influenza a Virus Activity Exerted by Baicalein and Biochanin A. BMC Res. Notes 7, 384. 10.1186/1756-0500-7-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisy D., Avilov S. V., Jacob Y., Laoide B. M., Ge X., Baudin F., et al. (2012). HMGB1 Protein Binds to Influenza Virus Nucleoprotein and Promotes Viral Replication. J. Virol. 86 (17), 9122–9133. 10.1128/jvi.00789-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Moriguchi R., Suzuki Y., Tomimori T., Yamada H. (1995a). Mode of Action of the Anti-influenza Virus Activity of Plant Flavonoid, 5,7,4′-Trihydroxy-8-Methoxyflavone, from the Roots of Scutellaria Baicalensis. Antiviral Res. 26 (1), 11–25. 10.1016/0166-3542(94)00062-d [DOI] [PubMed] [Google Scholar]
- Nagai T., Suzuki Y., Tomimori T., Yamada H. (1995b). Antiviral Activity of Plant Flavonoid, 5,7,4'-Trihydroxy-8-Methoxyflavone, from the Roots of Scutellaria Baicalensis against Influenza A (H3N2) and B Viruses. Biol. Pharm. Bull. 18 (2), 295–299. 10.1248/bpb.18.295 [DOI] [PubMed] [Google Scholar]
- Nayak M. K., Agrawal A. S., Bose S., Naskar S., Bhowmick R., Chakrabarti S., et al. (2014). Antiviral Activity of Baicalin against Influenza Virus H1N1-Pdm09 Is Due to Modulation of NS1-Mediated Cellular Innate Immune Responses. J. Antimicrob. Chemother. 69 (5), 1298–1310. 10.1093/jac/dkt534 [DOI] [PubMed] [Google Scholar]
- Oo A., Rausalu K., Merits A., Higgs S., Vanlandingham D., Bakar S. A., et al. (2018). Deciphering the Potential of Baicalin as an Antiviral Agent for Chikungunya Virus Infection. Antiviral Res. 150, 101–111. 10.1016/j.antiviral.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Orzechowska B., Chaber R., Wiśniewska A., Pajtasz-Piasecka E., Jatczak B., Siemieniec I., et al. (2014). Baicalin from the Extract of Scutellaria Baicalensis Affects the Innate Immunity and Apoptosis in Leukocytes of Children with Acute Lymphocytic Leukemia. Int. Immunopharmacology 23 (2), 558–567. 10.1016/j.intimp.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Ou C., Zhang Q., Wu G., Shi N., He C. (2015). Dryocrassin ABBA, a Novel Active Substance for Use against Amantadine-Resistant H5N1 Avian Influenza Virus. Front. Microbiol. 6, 592. 10.3389/fmicb.2015.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang P., Zheng K., Wu S., Xu H., Deng L., Shi Y., et al. (2018). Baicalin Downregulates RLRs Signaling Pathway to Control Influenza A Virus Infection and Improve the Prognosis. Evid. Based Complement. Alternat Med. 2018, 4923062. 10.1155/2018/4923062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penarrubia A. L., Ruiz M., Porco R., Rao S. N., Vella S. A., Juanola-Falgarona M., et al. (2020). Multiple Assays in a Real-Time RT-PCR SARS-CoV-2 Panel Can Mitigate the Risk of Loss of Sensitivity by New Genomic Variants during the COVID-19 Outbreak. Int. J. Infect. Dis. 97, 225-229. 10.1016/j.ijid.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S. -H. (2016). An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J. Med. Chem. 59 (14), 6595–6628. 10.1021/acs.jmedchem.5b01461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A., Yost J., Etxeandia-Ikobaltzeta I., Abraham G. M., Jokela J. A., Forciea M. A., et al. (2021). Should Remdesivir Be Used for the Treatment of Patients with COVID-19? Rapid, Living Practice Points from the American College of Physicians (Version 2). Ann. Intern. Med. M208101. 10.7326/m20-8101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Fang J.-g., Xiao J., Liu T., Liu J., Zhang Y.-l., et al. (2013). Effect of Baicalein on the Expression of VIP in Extravillous Cytotrophoblasts Infected with Human Cytomegalovirus In Vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 33 (3), 406–411. 10.1007/s11596-013-1132-9 [DOI] [PubMed] [Google Scholar]
- Qu X.-y., Li Q.-j., Zhang H.-m., Zhang X.-j., Shi P.-h., Zhang X.-j., et al. (2016). Protective Effects of Phillyrin against Influenza A Virus In Vivo. Arch. Pharm. Res. 39 (7), 998–1005. 10.1007/s12272-016-0775-z [DOI] [PubMed] [Google Scholar]
- Ren X. H., Qi X., Zuo Q., Tang J., Liu D. (2020). Analysis of Treatment of 813 COVID-19 Patients in the Fangcang Hospital. Med. Guide 39 (07), 926–930. 10.3870/j.issn.1004-0781.2020.07.008 [DOI] [Google Scholar]
- Roy S., He R., Kapoor A., Forman M., Mazzone J. R., Posner G. H., et al. (2015). Inhibition of Human Cytomegalovirus Replication by Artemisinins: Effects Mediated through Cell Cycle Modulation. Antimicrob. Agents Chemother. 59 (7), 3870–3879. 10.1128/aac.00262-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., et al. (2020). Lianhuaqingwen Exerts Anti-viral and Anti-inflammatory Activity against Novel Coronavirus (SARS-CoV-2). Pharmacol. Res. 156, 104761. 10.1016/j.phrs.2020.104761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong R.-K., Kim J.-A., Shin O. S. (2018). Wogonin, a Flavonoid Isolated from Scutellaria Baicalensis, Has Anti-viral Activities against Influenza Infection via Modulation of AMPK Pathways. Acta Virol. 62 (1), 78–85. 10.4149/av_2018_109 [DOI] [PubMed] [Google Scholar]
- Shen C., Zhang Z., Xie T. (2020). Rhein Suppresses Lung Inflammatory Injury Induced by Human Respiratory Syncytial Virus through Inhibiting NLRP3 Inflammasome Activation via NF-Κb Pathway in Mice. Front. Pharmacol. 10, 1600. 10.3389/fphar.2019.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-c., Zhu H.-y., Li H., Zeng D.-l., Shi X.-l., Zhang Y.-y., et al. (2020). Regulating the Balance of Th17/Treg Cells in Gut-Lung axis Contributed to the Therapeutic Effect of Houttuynia Cordata Polysaccharides on H1N1-Induced Acute Lung Injury. Int. J. Biol. Macromolecules 158, 52–66. 10.1016/j.ijbiomac.2020.04.211 [DOI] [PubMed] [Google Scholar]
- Sithisarn P., Michaelis M., Schubert-Zsilavecz M., Cinatl J., Jr. (2013). Differential Antiviral and Anti-inflammatory Mechanisms of the Flavonoids Biochanin A and Baicalein in H5N1 Influenza A Virus-Infected Cells. Antiviral Res. 97 (1), 41–48. 10.1016/j.antiviral.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Swarup V., Ghosh J., Mishra M. K., Basu A. (2008). Novel Strategy for Treatment of Japanese Encephalitis Using Arctigenin, a Plant Lignan. J. Antimicrob. Chemother. 61 (3), 679–688. 10.1093/jac/dkm503 [DOI] [PubMed] [Google Scholar]
- Trivedi A., Sharma S., Ashtey B. (2020). Investigational Treatments for COVID-19. Pharm. J. 304 (7938). 10.1211/PJ.2020.20208051 [DOI] [Google Scholar]
- Walls A. C., Park Y.-J., Tortorici M. A., Wall A., McGuire A. T., Veesler D. (2020). Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181 (2), 281–292. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q., et al. (2015). Effects of Baicalin on ET-1 and its Receptors of Pneumonia Mice Lung Tissue Infected with Influenza A Virus. Chin. J. Traditional Chin. Med. Pharm. 30 (4), 1290–1293. [Google Scholar]
- Wan Q., Wang H., Han X., Lin Y., Yang Y., Gu L., et al. (2014). Baicalin Inhibits TLR7/MYD88 Signaling Pathway Activation to Suppress Lung Inflammation in Mice Infected with Influenza A Virus. Biomed. Rep. 2 (3), 437–441. 10.3892/br.2014.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., et al. (2020). Characteristics of Lymphocyte Subsets and Cytokines in Peripheral Blood of 123 Hospitalized Patients with 2019 Novel Coronavirus Pneumonia (NCP). Preprint at https://www.medrxiv.org/content/10.1101/2020.02.10.20021832v1 (2020). [Google Scholar]
- Wang H., Ding Y., Zhou J., Sun X., Wang S. (2009a). The In Vitro and In Vivo Antiviral Effect of Salidroside and its Analogue against Coxsackievirus B3. Chin. J. Hosp. Pharm. 29 (18), 1514–1518. [Google Scholar]
- Wang J., Chen X., Wang W., Zhang Y., Yang Z., Jin Y., et al. (2013). Glycyrrhizic Acid as the Antiviral Component of Glycyrrhiza Uralensis Fisch. Against Coxsackievirus A16 and Enterovirus 71 of Hand Foot and Mouth Disease. J. Ethnopharmacology 147 (1), 114–121. 10.1016/j.jep.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. (2020b). Review of the 2019 Novel Coronavirus (SARS-CoV-2) Based on Current Evidence. Int. J. Antimicrob. Agents 55 (6), 105948. 10.1016/j.ijantimicag.2020.105948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.-J., Yang C.-H., Jin Y., Wan C.-B., Qian W.-H., Xing F., et al. (2020c). Baicalin Inhibits Coxsackievirus B3 Replication by Reducing Cellular Lipid Synthesis. Am. J. Chin. Med. 48 (1), 143–160. 10.1142/s0192415x20500081 [DOI] [PubMed] [Google Scholar]
- Wang Q.-W., Su Y., Sheng J.-T., Gu L.-M., Zhao Y., Chen X.-X., et al. (2018). Anti-influenza A Virus Activity of Rhein through Regulating Oxidative Stress, TLR4, Akt, MAPK, and NF-Κb Signal Pathways. PLoS One 13 (1), e0191793. 10.1371/journal.pone.0191793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-Y., Tseng C.-P., Tsai K.-C., Lin C.-F., Wen C.-Y., Tsay H.-S., et al. (2009b). Bioactivity-guided Screening Identifies Pheophytin a as a Potent Anti-hepatitis C Virus Compound from Lonicera Hypoglauca Miq. Biochem. Biophysical Res. Commun. 385 (2), 230–235. 10.1016/j.bbrc.2009.05.043 [DOI] [PubMed] [Google Scholar]
- Wang T., Wang X., Zhuo Y., Si C., Yang L., Meng L., et al. (2020a). Antiviral Activity of a Polysaccharide from Radix Isatidis (Isatis Indigotica Fortune) against Hepatitis B Virus (HBV) In Vitro via Activation of JAK/STAT Signal Pathway. J. Ethnopharmacology 257, 112782. 10.1016/j.jep.2020.112782 [DOI] [PubMed] [Google Scholar]
- Wang W., Xu S., Guo K., Xu J., Cheng G., Yu J., et al. (2010). Effects of Herba Agastachis Essential Oil and Cortex Phellodendri Alkaloid on the Antioxidation of IEC-6 in High-Temperature. Chin. J. Vet. Med. 46 (7), 60–64. 10.3969/j.issn.0529-6005.2010.07.028 [DOI] [Google Scholar]
- Wei W., Du H., Shao C. (2019). Screening of Antiviral Components of Ma Huang Tang and Investigation on the Ephedra Alkaloids Efficacy on Influenza Virus Type A. Front. Pharmacol. 10, 961. 10.3389/fphar.2019.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Peng C., Wan F. (2013). Study on the Inhibitory Effect of Anti-respiratory Viruses and Toxicity of Patchouli Alcohol In Vitro. Pharmacol. Clin. Chin. Materia Med. 29 (1), 26–29. 10.13412/j.cnki.zyyl.2013.01.010 [DOI] [Google Scholar]
- Wei Z.-Y., Wang X.-B., Zhang H.-Y., Yang C.-H., Wang Y.-B., Xu D.-H., et al. (2011). Inhibitory Effects of Indigowoad Root Polysaccharides on Porcine Reproductive and Respiratory Syndrome Virus Replication In Vitro. Antivir. Ther. 16 (3), 357–363. 10.3851/imp1755 [DOI] [PubMed] [Google Scholar]
- Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O. H. (2009). Glycyrrhizin Inhibits Influenza A Virus Uptake into the Cell. Antiviral Res. 83 (2), 171–178. 10.1016/j.antiviral.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Jin Y., Wu J., Yu X., Hao Y. (2011). Effects of Wogonin on Inflammation-Related Factors in Alveolar Macrophages Infected with Influenza Virus. Chin. J. Pathophysiology 27 (3), 533–538. 10.3969/j.issn.1000-4718.2011.03.022 [DOI] [Google Scholar]
- Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., et al. (2020). Efficacy of Huoxiang Zhengqi Dropping Pills and Lianhua Qingwen Granules in Treatment of COVID-19: A Randomized Controlled Trial. Pharmacol. Res. 161, 105126. 10.1016/j.phrs.2020.105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P., Ye W., Chen J., Li X. (2016). Antiviral Activities against Influenza Virus (FM1) of Bioactive Fractions and Representative Compounds Extracted from Banlangen (Radix Isatidis). J. Tradit Chin. Med. 36 (3), 369–376. 10.1016/s0254-6272(16)30051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., He L., Chen J., Hou X., Fan F., Wu H., et al. (2019). Different Types of Effective Fractions from Radix Isatidis Revealed a Multiple-Target Synergy Effect against Respiratory Syncytial Virus through RIG-I and MDA5 Signaling Pathways, a Pilot Study to Testify the Theory of Superposition of Traditional Chinese Medicine Efficacy. J. Ethnopharmacology 239, 111901. 10.1016/j.jep.2019.111901 [DOI] [PubMed] [Google Scholar]
- Xu Y., Sun J., He S. (2010). Effect of Three Kinds of Radix Isatidis Preparation on the Expression of Nucleoprotein of Influenza Virus. Shandong Med. J. 50 (27), 8–10. [Google Scholar]
- Yang G., et al. (2010). Study on Inhibitory Effect of Five Kinds of Traditional Chinesel Medicine Including Dryopteris Crassirhizoma on Influenza A Virus FM1 Strain. J. Pract. Traditional Chin. Intern. Med. 24 (7), 3–4. [Google Scholar]
- Yang Q., Gao L., Si J., Sun Y., Liu J., Cao L., et al. (2013). Inhibition of Porcine Reproductive and Respiratory Syndrome Virus Replication by Flavaspidic Acid AB. Antiviral Res. 97 (1), 66–73. 10.1016/j.antiviral.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Yeh C., Wang K. C., Chiang L. C., Shieh D. E., Yen M. H., Chang J. (2013). Water Extract of Licorice Had Anti-viral Activity against Human Respiratory Syncytial Virus in Human Respiratory Tract Cell Lines. J. Ethnopharmacol 148 (2), 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., et al. (2020a). Efficacy Study of Arbidol, Qingfei Paidu Decoction, Lianhua Qingwen Capsule, and Jinye Baidu Granules in the Treatment of Mild/moderate COVID-19 in a Fangcang Shelter Hospital. Pharmacol. Clin. Chin. materia Med. 36 (6), 2–6. [Google Scholar]
- Yu P., et al. (2020b). Effects of Lianhua Qingwen Granules Plus Arbidol on Treatment of Mild Corona Virus Disease-19. Chin. Pharm. J. 55 (12), 1042–1045. [Google Scholar]
- Zhang L., et al. (2017). Effect of Active Extracts from Radix Isatidis against Respiratory Syncytial Virus In Vitro. Liaoning J. Traditional Chin. Med. 44 (5), 1007–1011. [Google Scholar]
- Zhang P., et al. (2018). Effect of Baicalin on the Expression of Type I Interferon and SOCS1/3 in Rats Infected with Respiratory Syncytial Virus. Chin. J. Traditional Chin. Med. 33 (01), 328–332. [Google Scholar]
- Zhang Q., Cao F., Wang Y., Xu X., Sun Y., Li J., et al. (2020). The Efficacy and Safety of Jinhua Qinggan Granule (JHQG) in the Treatment of Coronavirus Disease 2019 (COVID-19). Medicine (Baltimore) 99 (24), e20531. 10.1097/md.0000000000020531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu X., Liu X. (2009). Anti-Coxsackievirus B3 Effects of Rhodiola Sachalinensis Polysaccaride In Vitro. Chin. J. Hosp. Pharm. 29 (20), 1749–1753. [Google Scholar]
- Zhang Y., Wang H., Liu Y., Wang C., Wang J., Long C., et al. (2018). Baicalein Inhibits Growth of Epstein-Barr Virus-Positive Nasopharyngeal Carcinoma by Repressing the Activity of EBNA1 Q-Promoter. Biomed. Pharmacother. 102, 1003–1014. 10.1016/j.biopha.2018.03.114 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zheng M., Zhu Z., Zheng L., Qiu B., Cao H., et al. (2014). In Vitro Anti-respiratory Syncytial Virus Effect of the Extraction of Lonicera japonica Thunb. J. New Chinese Med. 46 (6), 204–206. 10.13457/j.cnki.jncm.2014.06.097 [DOI] [Google Scholar]
- Zheng W. K. (2020). SARS-CoV-2 Infection of Respiratory Tract. J. Traditional Chin. Med., 1–5. [Google Scholar]
- Zhi H.-J., Zhu H.-Y., Zhang Y.-Y., Lu Y., Li H., Chen D.-F. (2019). In vivo effect of Quantified Flavonoids-Enriched Extract of Scutellaria Baicalensis Root on Acute Lung Injury Induced by Influenza A Virus. Phytomedicine 57, 105–116. 10.1016/j.phymed.2018.12.009 [DOI] [PubMed] [Google Scholar]
- Zhong T., Zhang L.-y., Wang Z.-y., Wang Y., Song F.-m., Zhang Y.-h., et al. (2017). Rheum Emodin Inhibits Enterovirus 71 Viral Replication and Affects the Host Cell Cycle Environment. Acta Pharmacol. Sin 38 (3), 392–401. 10.1038/aps.2016.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., et al. (2017). Mechanism Study of Houttuynia Cordata Anti-herpes Simplex Virus. China Feed (10), 10–16. [Google Scholar]
- Zhou Z., Li X., Liu J., Dong L., Chen Q., Liu J., et al. (2015). Honeysuckle-encoded Atypical microRNA2911 Directly Targets Influenza A Viruses. Cell Res. 25 (1), 39–49. 10.1038/cr.2014.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Lu X., Ling L., Li H., Ou Y., Shi X., et al. (2018). Houttuynia Cordata Polysaccharides Ameliorate Pneumonia Severity and Intestinal Injury in Mice with Influenza Virus Infection. J. Ethnopharmacology 218, 90–99. 10.1016/j.jep.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Zhu M., Mao S., Liu Y., Wang L., Chen T., Qin L., et al. (2016). Study on the Antiviral Effect of Lonicera Japonica Water Decoction on Influenza Virus. Chin. Med. Mod. Distance Education China 14 (9), 135–137. 10.3969/j.issn.1672-2779.2016.09.059 [DOI] [Google Scholar]
- Zhu X., Li W. (2012). Study on the Antiviral Activity of Water Extract of Ephedra Sinica against Respiratory Syncytial Virus Infection In Vitro. Pract. Prev. Med. 19 (10), 1555–1557. 10.3969/j.issn.1006-3110.2012.10.044 [DOI] [Google Scholar]
- Zumla A., Hui D. S., Azhar E. I., Memish Z. A., Maeurer M. (2020). Reducing Mortality from 2019-nCoV: Host-Directed Therapies Should Be an Option. The Lancet 395 (10224), e35–e36. 10.1016/s0140-6736(20)30305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Rao M., Wallis R. S., Kaufmann S. H., Rustomjee R., Mwaba P., et al. (2015). Towards Host-Directed Therapies for Tuberculosis. Nat. Rev. Drug Discov. 14 (8), 511–512. 10.1038/nrd4696 [DOI] [PubMed] [Google Scholar]
- Zuo Y., Dai M., Wang Z., Liu J. (2008). Effects of Banlangen Polysaccharide on Mice Resistance to Influenza Virus Infection. West China J. Pharm. Sci. 23 (6), 666–667. 10.3969/j.issn.1006-0103.2008.06.015 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.