Abstract
Shared decision making in advanced chronic kidney disease (CKD) requires unbiased information on survival and person-centred outcomes known to matter to patients: quality of life, symptom burden and support from family and healthcare professionals. To date, when deciding between dialysis and conservative care, patients have had to rely on evidence from small observational studies. Clinicians recognize that like is not being compared with like in these studies, and interpret the results differently. Furthermore, support differs considerably between renal units. What patients choose therefore depends on which renal unit they attend. To address this, a programme of work has been underway in the UK. After reports on survival and symptoms from a small number of renal units, a national, mixed-methods study—the Conservative Kidney Management Assessment of Practice Patterns Study—mapped out conservative care practices and attitudes in the UK. This led to the Prepare for Kidney Care study, a randomized controlled trial comparing preparation for dialysis versus preparation for conservative care. Although powered to detect a positivist 0.345 difference in quality-adjusted life years between the two treatments, this trial also takes a realist approach with a range of person-centred secondary outcomes and embedded qualitative research. To understand generalizability, it is nested in an observational cohort study, which is nested in a CKD registry. Challenges to recruitment and retention have been rapidly identified and addressed using an established embedded mixed methods approach—the QuinteT recruitment intervention. This review considers the background to and progress with recruitment to the trial.
Keywords: conservative care, comparative effectiveness, dialysis, randomized controlled trial
INTRODUCTION
A range of integrated treatment options should be available for estimated 4.9–9.7 million people who develop end-stage kidney disease each year worldwide—haemodialysis (HD), peritoneal dialysis (PD), transplantation and conservative care [1]. Reliable, stratified evidence of outcomes associated with each of these modalities is needed, whether local culture dictates that it is the patient, the family or the physician making the choice. This article provides background to and presents the evolution of a programme of work underway in the UK to provide more robust evidence in one of these areas: the comparative effectiveness of dialysis and conservative care in the frail older people with multiple health conditions.
CHALLENGES IN COUNTING AND QUALITY ASSURING TREATMENT OF KIDNEY FAILURE
Renal registries provide quality assurance for kidney replacement therapy (KRT), but in the early weeks of treatment, ascertainment bias is a problem (so some early deaths will be missed) [2] and few registries include kidney failure treated without dialysis [3]. Due to a lack of consensus on definitions, this latter group has proven particularly difficult to count, but in high-income countries is estimated to be ∼15% of all patients known to kidney clinics [4, 5]. Less well studied is kidney failure not referred to kidney clinics, but routine data from Canada and Australia suggest that in the >85 years age group, there may be as many as 13 people not treated with KRT for everyone who is [6, 7].
Defining conservative care
Treatment of kidney failure without dialysis—conservative care—can be divided into three types: comprehensive conservative care, where treatment is chosen or medically advised; choice-restricted conservative care, where resource constraints limit access to KRT; and unrecognized Stage 5 chronic kidney disease (CKD) [8]. The first of these, comprehensive conservative care, is most relevant to this review and is defined as ‘planned holistic patient-centred care for patients with stage 5 CKD and including a full range of treatment and support, but not dialysis’ [8].
So, if comprehensive conservative care is patient-centred, we must consider what matters to patients approaching the end of their lives. In a European, seven-country, telephone survey of >9000 people (median age 50 years, interquartile rage 40–62), most prioritized quality of life over survival (from 57% in Italy to 81% in Spain), including those with advanced illness [9]. In this study, only 2–6% of patients in countries said that extending life was most important, and this did not vary according to the respondent’s health status [9]. More specifically, a systematic review of the literature by Parker et al. found that while survival does matter to those with advanced life-limiting illness, equally and sometimes more important are: improved quality of life; control of pain and other symptoms; family support; knowing what to expect and having time to prepare; knowing that—if their disease is not curable and is deteriorating—the professionals are comfortable talking about death and dying; and continuity and co-ordination of care [10].
To help patients make shared decisions as they approach kidney failure, it is therefore important to have data on a wide range of these outcomes—survival, quality of life and symptoms, and more—and tailor the information provided to the priorities of the individual. Comparing experiences on dialysis versus conservative care brings unique challenges, however, and these must be appreciated when providing information to patients. Studies tend to be small, retrospective and observational, with all the associated biases [11]. Particular challenges are biased between the groups compared (where there are unmeasured factors that determine which treatment the patient receives, i.e. confounding by indication), and lead-time bias (where it is hard to identify when dialysis would have been started in patients on a conservative care pathway [12]).
Evidence on comparative effectiveness of dialysis and conservative care
Two systematic reviews have looked at quality of life and symptoms in relation to dialysis and conservative care [13, 14]. In individual studies, physical quality of life has been shown to be significantly lower in patients choosing conservative care rather than dialysis, though mental quality of life is similar [15–17]; a nice reminder that we are not comparing like with like in the observational studies. Once on their chosen treatments, trends in quality of life are broadly similar, though dialysis initiation tends to be associated with a reduction in ‘satisfaction with life’ [17] and an increase in ‘effect of kidney disease’ and ‘burden of kidney disease’ [18].
Prognosis is still an important consideration [9, 10, 19]. While there is clear evidence that, on average, those aged >75 years treated with KRT can expect to live longer than those managed conservatively [20], this survival advantage diminishes when patients have higher comorbidity and poor functional status. A systematic review of older people (mean age across the studies ranged from 60.5 to 92.0 years) treated with dialysis or conservative care found 89 studies conducted between 1976 and 2014 including 294 921 older people with kidney failure. Regardless of whether patients were managed conservatively or with dialysis, initial 1-year survival was similar at 73% [95% confidence interval (CI) 66–80%] in the dialysis group and 71% (95% CI 63–78%) in the conservatively managed group. At 2 years, however, the data suggest a survival advantage in the group managed with dialysis: 62% (95% CI 55–69%) for the dialysis group and 44% (95% CI 36–53%) for those managed conservatively. While dialysis initiation may be associated with additional early risk, we should not over-interpret these observational data as it is likely that the group of patients with a better prognosis will have been chosen (or been medically advised to choose) to have dialysis. Even novel statistics can only adjust for measured confounders; random allocation of treatment groups is required if groups are to be balanced for measured and unmeasured confounders [11]. There was also residual heterogeneity in the survival data, both between the studies and within the treatment groups. This likely reflects the long period covered by the review, the changes in treatment rates over time, differences in primary care referral patterns and differences in the components of care provided by centres [20]. Finally, it should also be noted that only 724 (0.2%) of the 294 921 people studies received conservative care and that only six studies directly compared survival on the two treatments.
The Conservative Kidney Management Assessment of Practice Patterns Study
Although conservative care was being provided in the UK in the early-2000s, the components provided and resources available varied greatly [21]. Guidance from the Department of Health [22, 23] and kidney community [24–26] created a framework for delivering higher quality conservative care in the mid-2000s and it was in this context that the Conservative Kidney Management Assessment of Practice Patterns Study (CKMAPPS) was conceived and funded by the National Institute for Health Research. Its purpose, stated from the beginning, was twofold: to inform service development and design of a future prospective multicentre study to evaluate the effectiveness, cost-effectiveness and appropriateness of conservative care compared with dialysis for treating elderly patients [27].
As a ‘complex intervention’ [28], conservative care needed to be better understood before a future definitive study could be undertaken. CKMAPPS therefore adopted a mixed-methods approach, with five sub-studies—a patient interview study, a staff interview study, a survey of renal units, a general practitioner interview study, and a data linkage exercise [27]; the first three of these playing a key part in informing the design of Prepare for Kidney Care. The patient interviews highlighted the contrasting beliefs held by older patients according to the treatment option they have chosen and the renal unit they have attended [29]. It called for better evidence about the effectiveness of conservative care to support shared decision making [29]. In the staff interviews, most people stated that it was difficult to assess whether patients were suitable for renal replacement therapy or conservative care. While many staff considered it important for patients and their family to make their own decisions based on the information they had been given, some adopted a more directed approach and guided patients towards a decision, particularly if they felt that patients would not benefit from dialysis. Staff recognized that some patients changed their minds over time, supporting the theory that decision-making in this context is a process rather than a one-off event [27].
The patient and staff interviews also determined the content of the renal unit survey [30]. This revealed that, although erythropoietin, iron and symptom management were components of almost all conservative care pathways, 40% of renal units reported no psychology or social services support, and 50% could not offer home visits [30]. Most renal units (86%) reported discussing the option of conservative care with all patients at the age of >75 years and most (83%) reported using decision aids when discussing conservative care. Once patients decided to have conservative care or dialysis, all renal units reported that they subsequently reviewed that decision [27, 30].
As data were not available on the numbers of patients opting for conservative care in the UK, the survey asked clinical directors to estimate the percentage of the patients >75 years choosing conservative care in their unit, with striking differences reported. In 7 of the 42 responding renal units, <10% of patients were thought to be choosing conservative care, whereas in another six this figure was >80% (Figure 1) [27]. Although based on the reports of clinical directors rather than data, this raised the possibility of considerable geographic variation in what patients were choosing, based on which kidney unit they attended. While we could not be certain of the extent of the variation, nor how much of the variation was warranted [31], it seemed clear that equity in provision would require better evidence to inform clinicians’ judgements and (if organizational change was needed) to inform business cases.
FIGURE 1.
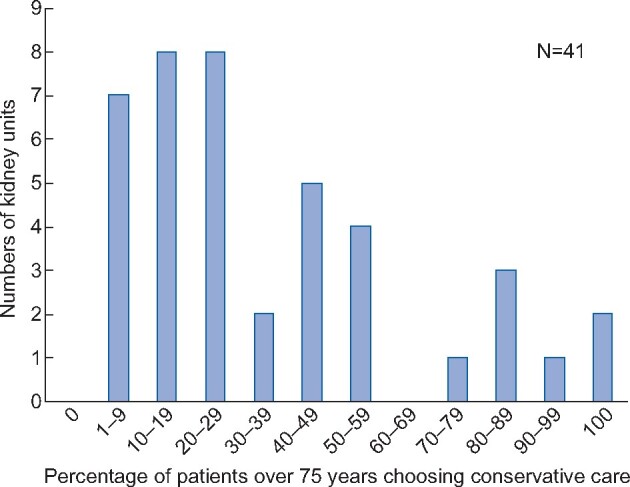
Percentage of patients with Stage 5 CKD choosing conservative care based on a survey of clinical directors asked the question: ‘In the calendar year 2012, what percentage of CKD5 patients aged 75+ years opted for conservative care?’ [27].
Beyond CKMAPPS: observational study or RCT?
The final question in the CKMAPPS survey explored clinical directors’ willingness to support future research in this area. After a steer from the funder and evidence from the ProtecT study that an embedded mixed-methods recruitment intervention could make challenging trials a success [32, 33], this final question asked about a future randomized controlled trial (RCT) as well as the originally intended observational study. The responses surprised many on the steering committee. When asked if their unit would consider entering a patient aged ≥75 years with Stage 5 CKD into an RCT comparing conservative care with dialysis, more than half of clinical directors (42 of 65, 65%) indicated that they would, for selected patients [27]. Of those, 18 units said that their unit would definitely be willing to participate in such a trial [27]. Only one unit reported they would be unwilling to participate in such a trial. Responses to a similar question about willingness to participate in an observational study were 60 of 65 (92%), 28 and 0, respectively [27].
Initial criticism of the RCT approach
There have been two major criticisms of adopting an RCT approach to address this question. One raised from the very beginning was whether it was ethical to randomly allocate patients to prepare for dialysis or conservative care. The other, which surfaced later and was less clearly expressed, related to whether the evidence from an RCT approach was going to be useful for decision making in such an individualized situation.
The ethics question turned out to be quite easily addressed. First, the observational data showed no clear survival or quality of life differences between patients choosing one treatment and patients choosing the other. Recognizing that treatment response is determined by more than a person’s age or list of diagnoses, the clinical teams also had to confirm that neither option—dialysis or conservative care—was inappropriate for a particular patient before they were offered information about the study. In addition, patients needed to have the mental capacity to make a shared decision to take part in the RCT and, importantly, could only remain on a treatment allocated through randomization if they retained that mental capacity. As with any RCT, patients were free to decide to come off their allocated treatment and return to usual care at any point after randomization.
The second criticism is an often-cited criticism of RCTs (and indeed observational studies) and relates to the way the results are presented as net effects and applied as ‘evidence-based medicine’ [34]. Indeed this had been a criticism of science since the 19th century, as it tried to predict and explain all actions and reactions—‘positivism’ [35]. In clinical research, this manifests as results being presented as net, population-average effects, ignoring individual differences. An extreme example of this is explanatory trials done by the pharmaceutical industry, but epidemiological analyses of observational data do the same thing. The alternative approach is a ‘realist’ one, which places greater emphasis on the range of outcomes that patients themselves prioritize, and embeds qualitative research to understand pathways and differences in responses and preferences [34].
While Prepare for Kidney Care is powered to detect a ‘positivist’ 0.345 quality-adjusted life years (QALYs) difference between the two treatment arms, its extensive qualitative work, numerous patient-centred secondary outcomes and embedded position within a registry cohort study allows for a realist approach to interpreting the results and incorporating them in future shared decision-making. Furthermore, a logic model was developed setting out how each of the components of the intervention might, it was hypothesized, produce the desired outcome. This logic model served two purposes: it informed decisions about the components of the intervention that were essential and the ones which could be delivered more flexibly by sites; and it determined the process data items that would need to be collected to assess fidelity of delivery of the interventions and explore, post hoc, how the intervention had had its effect (or not) [36]. In essence, the RCT approach is providing two groups of patients who should be similar in terms of measured and unmeasured confounders, allowing an unbiased comparison of the patient experience across a range of outcomes, something that is impossible to achieve with observational data [11].
Prepare for Kidney Care: the study design
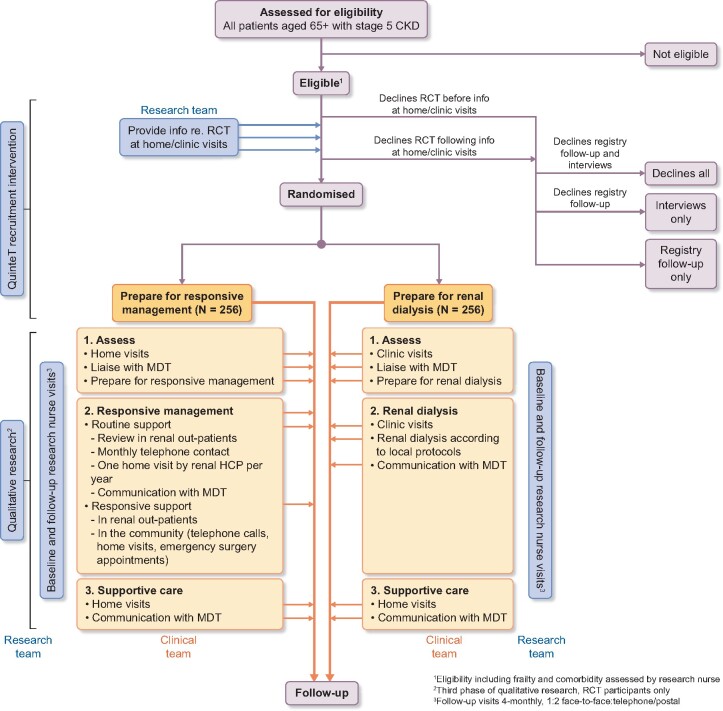
The main study is a two-arm, superiority, parallel group, non-blinded, individual-level, pragmatic RCT in multi-morbid, frail, older people with advanced CKD, comparing the QALYs gained >3 years after preparing for responsive management versus preparing for dialysis. The term responsive management was adopted for the conservative care arm for several reasons. There was feedback from QuinteT team that the word conservative may deter some patients, as it may not sound active enough. This had previously been an issue in the ProtecT trial [32]. It was also felt important that a distinction was made between conservative care as delivered locally and the protocol-determined conservative care (including home visits) being delivered as part of the trial. This RCT is embedded in an observational cohort study, which is itself embedded in a national Stage 4/5 CKD registry (Figure 2). Full details of the trial—including the power calculation and how enrolment works—are available from the publically available protocol [37] and will be published as a protocol paper before recruitment ends.
FIGURE 2.
Flow diagram for the Prepare for Kidney Care study. MDT, multidisciplinary team; HCP, healthcare professional.
Qualitative and mixed-methods are integrated throughout the trial to optimize its design and delivery. These are arranged in three interconnected stages: optimizing the trial design and intervention (Stage 1); optimizing recruitment (Stage 2); and understanding the acceptability of the intervention and reasons for non-compliance (Stage 3).
Someone will be eligible for the study if they have new or existing Stage 5 CKD and are:
aged 65+ years with a World Health Organization performance status 3+ [38], or
aged 65+ with a Davies comorbidity score 2+ [39], or
aged 80+ [38].
Although almost all of the observational evidence comparing conservative kidney care and dialysis is based on people 70 years and above, it was recognized by the trial management group that there are patients aged 65–70 years for whom conservative care is considered clinically appropriate. For this reason, and to ensure that the results of the trial addressed as many questions as possible in relation to the provision of dialysis and conservative care, it was agreed that for each person meeting these criteria, the local clinical team must agree that neither preparing for dialysis nor conservative care would inappropriate. Patients are considered ineligible if unable to consent, considered medically unfit for dialysis, within 4 weeks of needing to start dialysis, have had a previous kidney transplant, are ‘active’ on the kidney transplant waiting list or are being worked up for the kidney transplant waiting list.
Family members, friends and carers are also being invited to take part in a parallel study to assess carer burden.
The intervention and standard of care
A pragmatic approach was needed when designing the intervention and agreeing the standard of care. Pre-trial qualitative research was rapidly conducted with healthcare professionals to understand current practice and feasibility of the proposed intervention, as well as anticipated recruitment issues. Experts in conservative care and dialysis met to agree the core components and worked with the QuinteT team to present these in a balanced way [40]. The nomenclature of the two treatment options was carefully considered to minimize any influence on recruitment.
For presentation purposes, both treatment options were divided into three stages—an assess stage, a treatment stage and a supportive stage (Table 1).
Table 1.
The two treatment options in Prepare for Kidney Care
| Prepare for renal dialysis | Prepare for responsive management |
|---|---|
|
|
|
|
| Supportive care: as delivered locally | Supportive care: as delivered locally |
MDT, multidisciplinary team; HCP, healthcare professional.
For patients allocated to prepare for responsive management, the assess stage involves up to three home visits to undertake advanced care planning. Once this is considered complete, the patient progresses to the responsive management stage: clinic visits as usual, an annual home visit to reassess the advance care plan and a review of symptoms by telephone in any month that they did not have a clinic visit or home visit. Symptoms are managed optimally with medication, but if things progress to a stage where they cannot be controlled then the patient progresses to the supportive stage and has palliative care, as best delivered locally.
For patients allocated to prepare for renal dialysis, the assess stage involves coming to clinic as usual and agreeing the most appropriate way to prepare for dialysis. This can include plans to start HD with an arteriovenous fistula, graft or central venous catheter or have PD. If and when kidney function and symptoms progress to the point that dialysis is being considered, the decision to start is made by the treating clinician in agreement with the patient. Dialysis can be started incrementally. If symptoms cannot be controlled on dialysis, the patient can choose to progress to the supportive stage and receive palliative care, as best delivered locally.
It is worth noting that not all patients will have a progressive decline of the kidney function and need to decide to start dialysis during the study—we anticipate significant numbers will have stable function, decline slowly or die from other causes.
Understanding recruitment
The QuinteT Recruitment Intervention (QRI) was embedded into Prepare for Kidney Care to support trial recruitment [33]. Recruitment ‘lessons learned’ are often reported towards the end stages of an RCT, by which point there is limited opportunity to support accrual to the trial in question. The QRI is designed to rapidly investigate and address recruitment issues in real-time, using methodology that originated in the ProtecT study [41, 42]. The methods have been refined through application to >40 RCTs [43, 44], with Prepare for Kidney Care the first to apply these in a renal context.
Challenges and actions taken to address
As anticipated, recruitment to Prepare for Kidney Care has been no easy feat. Challenges identified through the QRI have varied across individual sites and over time, though several core issues have emerged:
some recruiters have been reluctant to discuss the trial with patients deemed to have a treatment plan in place, leading to around half of eligible RCT patients not being approached. This issue is linked to renal units’ tendencies to initiate discussions about end-stage CKD management before estimated glomerular filtration rate falls below 15, with the intention of supporting shared decision-making around future treatment. Recruiters’ reluctance and discomfort around discussing the trial with these eligible patients has stemmed from assumptions that decisions are fixed, and concerns that re-opening discussions could be confusing or distressing for patients. The time and resources invested into supporting patients are also at odds with the RCT recruitment discussion, which requires communication of uncertainty and equipoise [45], and
where eligible patients have been approached, audio-recordings of recruitment discussions have revealed a tendency for equipoise to be undermined through subtle indications that one treatment arm (often response management) is more appropriate than the other (preparation for renal dialysis).
Developing a nuanced understanding of recruitment issues has allowed for tailored actions to be implemented iteratively as sites have continued to open to recruitment. To address concerns around re-opening treatment discussions, clinical vignettes of patients who have entered the trial and extracts from audio-recorded discussions have been used to gently challenge assumptions that patients have fixed decisions. Challenges in conveying equipoise have been addressed through individual feedback for recruiters who provide audio-recordings of recruitment discussions, as well as study-wide ‘tips and guidance’ documents and ‘cue cards’ to support communication. These documents have been kept under review to ensure relevance as new evidence of recruitment issues emerge. The above strategies have been reinforced through investigator meetings, which have served as an opportunity to engender a collaborative approach to delivering Prepare for Kidney Care. The QRI strategies will continue through a combination of site-specific and study-wide activities until the end of recruitment.
Progress with recruitment
Prepare for Kidney Care was proposed to the Health Technology Assessment programme of the National Institute for Health Research and funded in September 2016. Work officially commenced in January 2017, with national research ethics approval secured in May 2017. Recognizing the complexity of the trial, site opening was arranged in two phases—an initial 4sites followed, 6 months later, by a further 12 sites. In fact, 6 sites opened in the initial phase and as of 31 March 2020, there are 24 sites open with 246 of the required 512 (48%) participants recruited. A further 103 participants have been recruited to the observational study. Additional funding has been secured from the funder to extend recruitment from the original end date of 31 September 2019 to 31 March 2021 and several additional sites are being worked up to open. However, the COVID-19 pandemic is likely to change some of these timelines, with recruitment activity paused from 1 April 2020 to allow resources to be focused on the immediate public health threat.
CONCLUSION
There have historically been few RCTs to inform practice in kidney clinics [46]. While there may be a number of explanations for this, other specialties have faced similar challenges and expanded and transformed the research they undertake. From a position in the mid-1990s, when evidence relied on case series and it was felt that ‘…the personal attributes that make a successful surgeon differ from those needed for collaborative multicentre research…’ [47], surgical colleagues have transformed the way they do research [48]. If we are to improve our evidence base and reduce unwarranted variation in practice, the kidney community must improve its ability to recognize uncertainty and offer randomization to patients. As one of the Prepare for Kidney Care investigators nicely put it when challenged: ‘I don’t see what the problem is, you are just offering patients a third option—they can choose to have dialysis, choose to have conservative care or choose to take part in research’. It is hard to argue with that.
ACKNOWLEDGEMENTS
The authors would like to thank all the patients who have agreed to take part in Prepare for Kidney Care, and their friends and families. They also acknowledge the extraordinary efforts of principal investigators, research nurses and clinical teams in the 24 sites; many have had to find local solutions to make recruitment possible in their local service (www.bristol.ac.uk/population-health-sciences/projects/prepare-kc-trial/recruitment-centres/). Finally, the authors would like to thank the Trial Management Group for their support and advice in delivering Prepare for Kidney Care. This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (project number 15/57/39). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Technology Assessment, NIHR, National Health Service or the Department of Health.
CONFLICT OF INTEREST STATEMENT
F.J.C. has received honoraria from Baxter and research funding from the National Institute for Health Research and Kidney Research UK. A.B. reports research costs having been received by her National Health Service Trust for the research.
(See related article by Clyne. Caring for older people with chronic kidney disease—primum non nocere. Nephrol Dial Transplant 2021; 36: 953–956)
REFERENCES
- 1. Harris DCH, Davies SJ, Finkelstein FO. et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int 2019; 95: S1–S33 [DOI] [PubMed] [Google Scholar]
- 2. Foley RN, Chen SC, Solid CA. et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014; 86: 392–398 [DOI] [PubMed] [Google Scholar]
- 3. Bello AK, Levin A, Lunney M. et al. Global Kidney Health Atlas: a report by the international society of nephrology on global burden of end stage kidney disease and capacity for kidney replacement therapy across world countries and regions. Brussels, Belgium: International Society of Nephrology, 2019
- 4. van de Luijtgaarden MW, Noordzij M, van Biesen W. et al. Conservative care in Europe–nephrologists' experience with the decision not to start renal replacement therapy. Nephrol Dial Transplant 2013; 28: 2604–2612 [DOI] [PubMed] [Google Scholar]
- 5. Morton RL, Howard K, Webster AC. et al. Patient information about options for treatment (PINOT): a prospective national study of information given to incident CKD Stage 5 patients. Nephrol Dial Transplant 2011; 26: 1266–1274 [DOI] [PubMed] [Google Scholar]
- 6. Hemmelgarn BR, James MT, Manns BJ. et al. Rates of treated and untreated kidney failure in older vs younger adults. JAMA 2012; 307: 2507–2515 [DOI] [PubMed] [Google Scholar]
- 7. Sparke C, Moon L, Green F. et al. Estimating the total incidence of kidney failure in Australia including individuals who are not treated by dialysis or transplantation. Am J Kidney Dis 2013; 61: 413–419 [DOI] [PubMed] [Google Scholar]
- 8. Davison SN, Levin A, Moss AH. et al. Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int 2015; 88: 447–459 [DOI] [PubMed] [Google Scholar]
- 9. Higginson IJ, Gomes B, Calanzani N. et al. Priorities for treatment, care and information if faced with serious illness: a comparative population-based survey in seven European countries. Palliat Med 2014; 28: 101–110 [DOI] [PubMed] [Google Scholar]
- 10. Parker SM, Clayton JM, Hancock K. et al. systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage 2007; 34: 81–93 [DOI] [PubMed] [Google Scholar]
- 11. Collins R, Bowman L, Landray M. et al. The magic of randomization versus the myth of real-world evidence. N Engl J Med 2020; 382: 674–678 [DOI] [PubMed] [Google Scholar]
- 12. Alston H, Burns A.. Conservative care of the patient with end-stage renal disease. Clin Med 2015; 15: 567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown L, Gardner G, Bonner A.. A comparison of treatment options for management of end stage kidney disease in elderly patients: a systematic review protocol. JBI Database Syst Rev Implement Rep 2013; 11: 12 [Google Scholar]
- 14. Ren Q, Shi Q, Ma T. et al. Quality of life, symptoms, and sleep quality of elderly with end-stage renal disease receiving conservative management: a systematic review. Health Qual Life Outcomes 2019; 17: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown MA, Collett GK, Josland EA. et al. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol 2015; 10: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Da Silva-Gane M, Wellsted D, Greenshields H. et al. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 2012; 7: 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Biase V, Tobaldini O, Boaretti C. et al. Prolonged conservative treatment for frail elderly patients with end-stage renal disease: the Verona experience. Nephrol Dial Transplant 2008; 23: 1313–1317 [DOI] [PubMed] [Google Scholar]
- 18. Seow YY, Cheung YB, Qu LM. et al. Trajectory of quality of life for poor prognosis stage 5D chronic kidney disease with and without dialysis. Am J Nephrol 2013; 37: 231–238 [DOI] [PubMed] [Google Scholar]
- 19. Steinhauser KE, Christakis NA, Clipp EC. et al. Preparing for the end of life: preferences of patients, families, physicians and other care providers. J Pain Symptom Manage 2001; 22: 727–737 [DOI] [PubMed] [Google Scholar]
- 20. Foote C, Kotwal S, Gallagher M. et al. Survival outcomes of supportive care versus dialysis therapies for elderly patients with end-stage kidney disease: a systematic review and meta-analysis. Nephrology (Carlton) 2016; 21: 241–253 [DOI] [PubMed] [Google Scholar]
- 21. Gunda S, Thomas M, Smith S.. National survey of palliative care in end-stage renal disease in the UK. Nephrol Dial Transplant 2005; 20: 392–395 [DOI] [PubMed] [Google Scholar]
- 22.Department of Health. End of Life Care Strategy. London: Department of Health, 2008
- 23.National Gold Standards Framework Centre. The Gold Standards Framework. 2014. http://www.goldstandardsframework.org.uk/ (23 March 2020, date last accessed)
- 24.Department of Health. National Services Framework - Part 2. London: Department of Health, 2005
- 25. Douglas C, Murtagh FE, Chambers EJ. et al. Symptom management for the adult patient dying with advanced chronic kidney disease: a review of the literature and development of evidence-based guidelines by a United Kingdom Expert Consensus Group. Palliat Med 2009; 23: 103–110 [DOI] [PubMed] [Google Scholar]
- 26.Department of Health. End of Life Care in Advanced Kidney Disease: A Framework for Implementation. London: Department of Health, 2009 [Google Scholar]
- 27. Roderick P, Rayner H, Tonkin-Crine S. et al. A National Study of Practice Patterns in UK Renal Units in the Use of Dialysis and Conservative Kidney Management to Treat People Aged 75 Years and Over with Chronic Kidney Failure. Southampton: NIHR Journals Library, 2015 [PubMed]
- 28. Craig P, Dieppe P, MacIntyre S. et al. Developing and Evaluating Complex Interventions: New Guidance. London:Medical Research Council, 2008 [DOI] [PMC free article] [PubMed]
- 29. Tonkin-Crine S, Okamoto I, Leydon GM. et al. Understanding by older patients of dialysis and conservative management for chronic kidney failure. Am J Kidney Dis 2015; 65: 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okamoto I, Tonkin-Crine S, Rayner H. et al. Conservative care for ESRD in the United Kingdom: a national survey. Clin J Am Soc Nephrol 2015; 10: 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sutherland K, Levesque JF.. Unwarranted clinical variation in health care: definitions and proposal of an analytic framework. J Eval Clin Pract 2019; 26: 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donovan J, Mills N, Smith M. et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: protect (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ (Clin Res Ed) 2002; 325: 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donovan JL, Rooshenas L, Jepson M. et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016; 17: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonell C, Moore G, Warren E. et al. Are randomised controlled trials positivist? Reviewing the social science and philosophy literature to assess positivist tendencies of trials of social interventions in public health and health services. Trials 2018; 19: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weber M, Shils E.. Max Weber on the Methodology of the Social Sciences. 1st edn. Glencoe, IL: Free Press, 1949, 188 [Google Scholar]
- 36.W.K. Kellogg Foundation. Using Logic Models to Bring Together Planning, Evaluation and Action: Logic Model Development Guide. Battle Creek, MI: W.K. Kellogg Foundation, 2004
- 37.Caskey FJ, Gibson A, Burns A, et al. The Prepare for Kidney Care Study.. https://www.journalslibrary.nihr.ac.uk/programmes/hta/155739/#/ (30 March 2020, date last accessed)
- 38. Hussain JA, Mooney A, Russon L.. Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 2013; 27: 829–839 [DOI] [PubMed] [Google Scholar]
- 39. Davies SJ, Phillips L, Naish PF. et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002; 17: 1085–1092 [DOI] [PubMed] [Google Scholar]
- 40. Husbands S, Caskey F, Winton H. et al. Pre-trial qualitative work with health care professionals to refine the design and delivery of a randomised controlled trial on kidney care. Trials 2019; 20: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Donovan JL, Hamdy FC, Lane JA. et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016; 375: 1425–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamdy FC, Donovan JL, Lane JA. et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–1424 [DOI] [PubMed] [Google Scholar]
- 43. Rooshenas L, Paramasivan S, Jepson M. et al. Intensive triangulation of qualitative research and quantitative data to improve recruitment to randomized trials: the quintet approach. Qual Health Res 2019; 29: 672–679 [DOI] [PubMed] [Google Scholar]
- 44. Rooshenas L, Scott LJ, Blazeby JM. et al. The quintet recruitment intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol 2019; 106: 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rooshenas L, Elliott D, Wade J. et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLoS Med 2016; 13: e1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palmer SC, Sciancalepore M, Strippoli GF.. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis 2011; 58: 335–337 [DOI] [PubMed] [Google Scholar]
- 47. Horton R. Surgical research or comic opera: questions, but few answers. Lancet 1996; 347: 984–985 [DOI] [PubMed] [Google Scholar]
- 48. Hirst A, Philippou Y, Blazeby J. et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg 2019; 269: 211–220 [DOI] [PubMed] [Google Scholar]